Exam 2: The Chemical Level of Organization
Exam 1: An Introduction to the Human Body78 Questions
Exam 2: The Chemical Level of Organization81 Questions
Exam 3: The Cellular Level of Organization84 Questions
Exam 4: The Tissue Level of Organization64 Questions
Exam 5: The Integumentary System78 Questions
Exam 6: The Skeletal System: Bone Tissue77 Questions
Exam 7: The Skeletal System: the Axial Skeleton80 Questions
Exam 8: The Skeletal System: The Appendicular Skeleton82 Questions
Exam 9: Joints41 Questions
Exam 10: Muscular Tissue85 Questions
Exam 11: The Muscular System70 Questions
Exam 12: Nervous Tissue17 Questions
Exam 13: The Spinal Cord and Spinal Nerves74 Questions
Exam 14: The Brain and Cranial Nerves72 Questions
Exam 15: The Autonomic Nervous System64 Questions
Exam 16: Sensory, Motor, and Integrative Systems76 Questions
Exam 17: The Special Senses73 Questions
Exam 18: The Endocrine System84 Questions
Exam 19: The Cardiovascular System: the Blood83 Questions
Exam 20: The Cardiovascular System: the Heart82 Questions
Exam 21: The Cardiovascular System: Blood Vessels and Hemodynamics81 Questions
Exam 22: The Lymphatic System and Immunity80 Questions
Exam 23: The Respiratory System85 Questions
Exam 24: The Digestive System85 Questions
Exam 25: Metabolism and Nutrition85 Questions
Exam 26: The Urinary System84 Questions
Exam 27: Fluid, Electrolyte, and Acidbase Homeostasis57 Questions
Exam 28: The Reproductive Systems84 Questions
Exam 29: Development and Inheritance83 Questions
Select questions type
The nucleus of unstable _____ of an element will decay leading to emission of radiation.
Free
(Multiple Choice)
4.7/5  (31)
(31)
Correct Answer:
D
Describe the functions of water in the body.
Free
(Essay)
4.9/5  (39)
(39)
Correct Answer:
Water is a Solvent that allows transportation of Solutes.Water acts in hydrolysis reactions to split reactants.Water can transport heat in the body and can be used to release heat from the body as occurs in sweating.Water is used as a lubricant,particularly in serous fluids like those surrounding the lungs and on mucosal membranes like those lining the gastrointestinal tract.
Which of the labeled structures are found in DNA but not RNA?
1 A
2 B
3 C
4 E
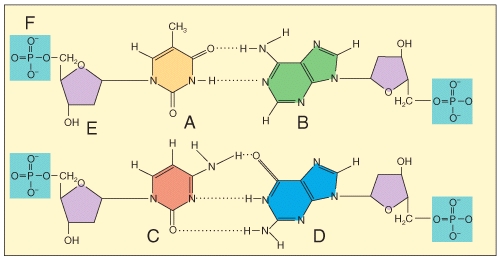
Free
(Multiple Choice)
4.9/5  (25)
(25)
Correct Answer:
E
A chemical compound that helps control the pH of a solution by adding or removing hydrogen ions is a(n)
(Multiple Choice)
4.7/5  (38)
(38)
AB + CD → AD + BC is a general example of a(n)_____ reaction.
(Multiple Choice)
4.7/5  (30)
(30)
Describe what happens to a protein's structure and function when it is denatured.
(Essay)
4.8/5  (29)
(29)
This type of lipid is the body's primary long-term energy storage molecule.
(Multiple Choice)
4.8/5  (38)
(38)
The chemical bonds formed between the oxygen and hydrogen atoms making up a water molecule are called
(Multiple Choice)
4.9/5  (29)
(29)
In the diagram,what pH value represents an acidic Solution?
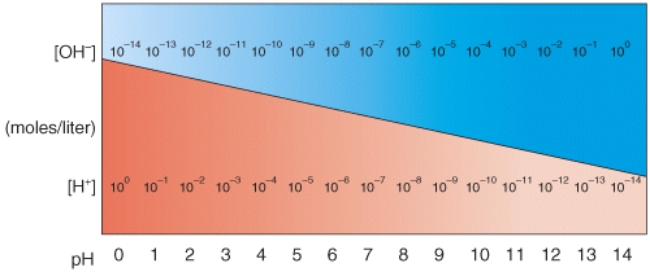
(Multiple Choice)
4.9/5  (31)
(31)
Choose the appropriate atomic number associated with the element.
Carbon's atomic mass (P = 6,N = 6)is [dropdown 1a].Carbon has [dropdown 2b] valence electrons.Carbon can form up to [dropdown 3c] covalent bonds.Carbon 14 is a radioactive isotope and contains [dropdown 4d] neutrons.
Dropdown choices
2
4
6
7
8
12
14
(Short Answer)
4.7/5  (32)
(32)
The energy stored in the bonds of the molecules in the foods that humans eat is
1)a form of kinetic energy.
2)a form of potential energy.
3)referred to as chemical energy.
(Multiple Choice)
4.8/5  (47)
(47)
What are the four major elements found in the chemicals that comprise the human body?
(Multiple Choice)
4.9/5  (26)
(26)
This refers to a weighted average of the atomic weights of all naturally occurring isotopes of an element.
(Multiple Choice)
4.8/5  (38)
(38)
A triple covalent bond is formed between atoms sharing _____ valence electrons.
(Multiple Choice)
4.7/5  (42)
(42)
In the body fluid compartments found in of the human body,the solvent is
(Multiple Choice)
4.9/5  (33)
(33)
Showing 1 - 20 of 81
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)