Deck 44: Nuclear Structure
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/50
Play
Full screen (f)
Deck 44: Nuclear Structure
1
Calculate the binding energy per nucleon (MeV/nucleon)for tritium, ( 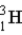
)a radioactive isotope of hydrogen.
Assume:
M p = 1.007 825 u
M n = 1.008 665 u
M t = 3.016 05 u
U = 1.66 × 10−27 kg
A)2.8
B)3.1
C)1.0
D)8.5
E)2.1
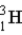
)a radioactive isotope of hydrogen.
Assume:
M p = 1.007 825 u
M n = 1.008 665 u
M t = 3.016 05 u
U = 1.66 × 10−27 kg
A)2.8
B)3.1
C)1.0
D)8.5
E)2.1
2.8
2
Two isotopes of uranium have the same
A)mass number
B)neutron number
C)atomic number
D)nucleon number
E)nucleon number and neutron number
A)mass number
B)neutron number
C)atomic number
D)nucleon number
E)nucleon number and neutron number
atomic number
3
The half-life of 131I is 8.04 days.Three days after it was prepared,its activity was 0.50 μCi.How many curies (in μCi)were initially prepared?
A).60
B).70
C).65
D).55
E).39
A).60
B).70
C).65
D).55
E).39
.65
4
What value of Z (atomic number)and A (mass number)result in the following β-decay? 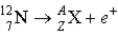
A)Z = 6;A = 12
B)Z = 5;A = 8
C)Z = 6;A = 11
D)Z = 8;A = 12
E)Z = 8;A = 11
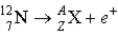
A)Z = 6;A = 12
B)Z = 5;A = 8
C)Z = 6;A = 11
D)Z = 8;A = 12
E)Z = 8;A = 11

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
5
How many radioactive atoms are present in a sample that has an activity of 0.5 μCi and a half-life of 10 years? (1 curie = 3.7 × 1010 decays/s)
A)9.5 × 1012 atoms
B)8.4 × 1012 atoms
C)7.3 × 1012 atoms
D)6.5 × 1012 atoms
E)2.7 × 105 atoms
A)9.5 × 1012 atoms
B)8.4 × 1012 atoms
C)7.3 × 1012 atoms
D)6.5 × 1012 atoms
E)2.7 × 105 atoms

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
6
Find the ratio of the binding energy per nucleon for helium ( 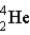
)to uranium-238 (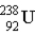
))
Assume:
M p = 1.007 825 u
M n = 1.008 665 u
MHe = 4.002 603 u
MU = 238.050 786 u
U = 1.66 × 10−27 kg
A)1.07
B)0.934
C)63.7
D)1.6 × 10−2
E)3.24
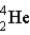
)to uranium-238 (
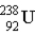
))
Assume:
M p = 1.007 825 u
M n = 1.008 665 u
MHe = 4.002 603 u
MU = 238.050 786 u
U = 1.66 × 10−27 kg
A)1.07
B)0.934
C)63.7
D)1.6 × 10−2
E)3.24

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
7
The ratio of the density of a neutron (r = r0A1/3)to the density of a classical electron (re = ke2/mec2 = 2.8 × 10−15 m)is
A)4.3 × 102
B)2.3 × 104
C)1.4 × 102
D)6.9 × 10−3
E)4.3 × 103
A)4.3 × 102
B)2.3 × 104
C)1.4 × 102
D)6.9 × 10−3
E)4.3 × 103

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
8
An alpha particle is emitted from a radioactive source with an energy of 5 MeV.How fast is it moving (in m/s)? (m = 4.002 603 u,1 u = 1.66 × 10−27 kg. )
A)2.4 × 107
B)1.6 × 107
C)3.7 × 107
D)4.6 × 107
E)2.1 × 107
A)2.4 × 107
B)1.6 × 107
C)3.7 × 107
D)4.6 × 107
E)2.1 × 107

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
9
44 g of petrified wood was found in a petrified forest.A sample showed a 14C activity of 100 decays/minute.How long has the tree been dead (in years)? (The half-life of carbon-14 is 5730 years and freshly cut wood contains 6.5 × 1010 atoms of 14C per gram. )
A)12300
B)15600
C)8500
D)4700
E)2400
A)12300
B)15600
C)8500
D)4700
E)2400

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
10
What value of Z (atomic number)and A (mass number)result in the following β-decay? 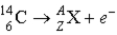
A)Z = 5;A = 14
B)Z = 4;A = 10
C)Z = 6;A = 14
D)Z = 7;A = 14
E)Z = 7;A = 13
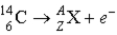
A)Z = 5;A = 14
B)Z = 4;A = 10
C)Z = 6;A = 14
D)Z = 7;A = 14
E)Z = 7;A = 13

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
11
The isotope,tritium,has a half-life of 12.3 years.Assume we have 10 kg of the substance.How much tritium will be left after 30 years?
A)0.20 kg
B)1.8 kg
C)0.18 kg
D)1.7 kg
E)4.1 kg
A)0.20 kg
B)1.8 kg
C)0.18 kg
D)1.7 kg
E)4.1 kg

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
12
What value of Z (atomic number)and A (mass number)result in the following alpha decay? 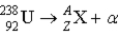
A)Z = 92;A = 238
B)Z = 91;A = 238
C)Z = 90;A = 234
D)Z = 93;A = 238
E)Z = 88;A = 236
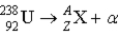
A)Z = 92;A = 238
B)Z = 91;A = 238
C)Z = 90;A = 234
D)Z = 93;A = 238
E)Z = 88;A = 236

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
13
The ratio of the radius of a classical electron (re = kee2/mec2 = 2.8 × 10−15 m)to the radius of a 4He nucleus (r = r0A1/3)is
A)2.0
B)0.68
C)1.5
D)0.92
E)2.4
A)2.0
B)0.68
C)1.5
D)0.92
E)2.4

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
14
Find the binding energy per nucleon (in MeV/nucleon)of carbon-12.
Assume:
MC = 12.000 000 u
M p = 1.007 825 u
M n = 1.008 665 u
U = 1.66 × 10−27 kg
A)1.2
B)4.2 × 10−2
C)7.4
D)7.7
E)5.6
Assume:
MC = 12.000 000 u
M p = 1.007 825 u
M n = 1.008 665 u
U = 1.66 × 10−27 kg
A)1.2
B)4.2 × 10−2
C)7.4
D)7.7
E)5.6

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
15
For large mass number nuclei which are stable,the ratio of protons to neutrons is
A)equal to 1
B)greater than 1
C)less than 1
D)unrelated to the stability of nuclei
E)almost 2 to 1
A)equal to 1
B)greater than 1
C)less than 1
D)unrelated to the stability of nuclei
E)almost 2 to 1

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
16
The isotope,tritium,has a half-life of 12.3 years.Assume we have 10 kg of the substance.What will be the disintegration constant (in s−1)?
A)5.6 × 10−2
B)5.6 × 108
C)3.2 × 107
D)1.8 × 10−9
E)1.6 × 106
A)5.6 × 10−2
B)5.6 × 108
C)3.2 × 107
D)1.8 × 10−9
E)1.6 × 106

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
17
Find the binding energy (in MeV)of carbon-12.
Assume:
MC = 12.000 000 u
M p = 1.007 825 u
M n = 1.008 665 u
U = 1.66 × 10−27 kg
A)14.8
B)0.511
C)9.11
D)92.3
E)46.2
Assume:
MC = 12.000 000 u
M p = 1.007 825 u
M n = 1.008 665 u
U = 1.66 × 10−27 kg
A)14.8
B)0.511
C)9.11
D)92.3
E)46.2

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
18
The isotope,tritium,has a half-life of 12.3 years.Assume we have 10 kg of the substance.What will be the initial decay rate,at t = 0 (in decays/s)?
A)1.09 × 1014
B)1.8 × 10−9
C)5.6 × 108
D)3.6 × 1018
E)3.6 × 1017
A)1.09 × 1014
B)1.8 × 10−9
C)5.6 × 108
D)3.6 × 1018
E)3.6 × 1017

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
19
Naturally radioactive nuclei can decay spontaneously by emitting the following particles:
A)helium nuclei,electrons,photons
B)electrons,neutrons,protons
C)helium nuclei,electrons,protons
D)electrons,neutrons,photons
E)quarks and leptons
A)helium nuclei,electrons,photons
B)electrons,neutrons,protons
C)helium nuclei,electrons,protons
D)electrons,neutrons,photons
E)quarks and leptons

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
20
The radius of a nucleus of 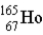
(in fm)is
A)15.4
B)5.5
C)12.8
D)6.6
E)4.9
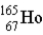
(in fm)is
A)15.4
B)5.5
C)12.8
D)6.6
E)4.9

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
21
It is often possible to use the atomic masses when calculating the binding energy of a nucleus.The reason for this is
A)the electron masses do not cancel.
B)the electron masses cancel.
C)tables of nuclear masses are usually not available.
D)the mass of the electron can usually be neglected when compared to the mass of the neutron.
E)the atomic masses are the same as the nuclear masses.
A)the electron masses do not cancel.
B)the electron masses cancel.
C)tables of nuclear masses are usually not available.
D)the mass of the electron can usually be neglected when compared to the mass of the neutron.
E)the atomic masses are the same as the nuclear masses.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
22
What value of Z (atomic number)and A (mass number)result in the following gamma decay? 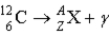
A)Z = 5;A = 12
B)Z = 4;A = 8
C)Z = 7;A = 12
D)Z = 6;A = 12
E)Z = 6;A = 11
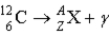
A)Z = 5;A = 12
B)Z = 4;A = 8
C)Z = 7;A = 12
D)Z = 6;A = 12
E)Z = 6;A = 11

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
23
The Q value for the following reaction, 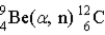
,is (in MeV)
M(alpha)= 4.002 603 u
M(Be)= 9.012 182 u
m(n.= 1.008 665 u
M(C)= 12.000 00 u
1 u = 1.66 × 10−27 kg
A)8.4
B)6.2
C)7.3
D)5.7
E)3.5
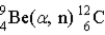
,is (in MeV)
M(alpha)= 4.002 603 u
M(Be)= 9.012 182 u
m(n.= 1.008 665 u
M(C)= 12.000 00 u
1 u = 1.66 × 10−27 kg
A)8.4
B)6.2
C)7.3
D)5.7
E)3.5

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
24
The radius of an approximately spherical nucleus is given by r =
A)r0Z3.
B)r0Z1/3.
C)r0A3.
D)r0A1/3.
E)r0(A − Z)1/3.
A)r0Z3.
B)r0Z1/3.
C)r0A3.
D)r0A1/3.
E)r0(A − Z)1/3.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
25
It is often possible to use atomic masses when calculating the binding energy of a nucleus.This is not true for calculating the Q value for the e+ decay process since
A)the electron masses do not cancel.
B)a positron is an antiparticle.
C)the electron masses cancel.
D)the mass of a positron cannot be neglected when compared to the mass of a nucleus.
E)none of the above.
A)the electron masses do not cancel.
B)a positron is an antiparticle.
C)the electron masses cancel.
D)the mass of a positron cannot be neglected when compared to the mass of a nucleus.
E)none of the above.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
26
Two nuclei which share the same atomic number Z always are
A)stable.
B)unstable.
C)isotopes.
D)isobars.
E)radioactive.
A)stable.
B)unstable.
C)isotopes.
D)isobars.
E)radioactive.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the effects listed below is not a major effect influencing the binding energy of the nucleus in the liquid-drop model?
A)The volume effect: the binding energy per nucleon is approximately constant when A > 50.
B)The surface effect: nucleons in the surface have fewer neighbors.
C)The quantum number effect: all nucleons in the nucleus have the same set of quantum numbers.
D)The Coulomb repulsion effect: protons repel protons.
E)The symmetry effect: stable nuclei tend to have N ≈ Z.
A)The volume effect: the binding energy per nucleon is approximately constant when A > 50.
B)The surface effect: nucleons in the surface have fewer neighbors.
C)The quantum number effect: all nucleons in the nucleus have the same set of quantum numbers.
D)The Coulomb repulsion effect: protons repel protons.
E)The symmetry effect: stable nuclei tend to have N ≈ Z.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
28
Because we know that the half-lives of many radioactive isotopes are millions of years,we can deduce that
A)the longer it exists the more radioactive nuclei Earth produces.
B)the sun is the source of all the radioactive nuclei on Earth.
C)there must have been many more radioactive nuclei on Earth when life began.
D)there must have been far fewer radioactive nuclei on Earth before life began.
E)the natural radioactivity of minerals on the Earth was created by the Earth's internal temperature.
A)the longer it exists the more radioactive nuclei Earth produces.
B)the sun is the source of all the radioactive nuclei on Earth.
C)there must have been many more radioactive nuclei on Earth when life began.
D)there must have been far fewer radioactive nuclei on Earth before life began.
E)the natural radioactivity of minerals on the Earth was created by the Earth's internal temperature.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
29
In beta decays
A)a proton changes to a neutron.
B)a neutron changes to a proton.
C)an electron is present in the nucleus before the decay.
D)(a), (b)or (c)may occur.
E)only (a)or (b)may occur.
A)a proton changes to a neutron.
B)a neutron changes to a proton.
C)an electron is present in the nucleus before the decay.
D)(a), (b)or (c)may occur.
E)only (a)or (b)may occur.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
30
Two nuclei may have equal Z,but different A,because they contain
A)equal numbers of protons and neutrons.
B)equal numbers of protons but different numbers of neutrons.
C)different numbers of protons but equal numbers of neutrons.
D)different numbers of protons and neutrons.
E)electrons as well as neutrons.
A)equal numbers of protons and neutrons.
B)equal numbers of protons but different numbers of neutrons.
C)different numbers of protons but equal numbers of neutrons.
D)different numbers of protons and neutrons.
E)electrons as well as neutrons.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
31
How can a nucleus be described by particular values of A,Z and N when the mass of the nucleus is not equal to Zmp + Nmn,where mp and mn are the masses of free protons and neutrons?
A)A,Z and N have no intrinsic meaning.
B)A,Z and N describe the number of particles of given types,but mass has no meaning when part of the mass is elsewhere in the universe.
C)A,Z and N describe the number of particles an ideal rather than a real nucleus would have.
D)A,Z and N describe the number of particles of given types in the nucleus,but not their masses in a bound state.
E)A,Z and N describe the number of particles of given types in the nucleus since the missing mass consists of electrons that are also present in the nucleus.
A)A,Z and N have no intrinsic meaning.
B)A,Z and N describe the number of particles of given types,but mass has no meaning when part of the mass is elsewhere in the universe.
C)A,Z and N describe the number of particles an ideal rather than a real nucleus would have.
D)A,Z and N describe the number of particles of given types in the nucleus,but not their masses in a bound state.
E)A,Z and N describe the number of particles of given types in the nucleus since the missing mass consists of electrons that are also present in the nucleus.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
32
The reaction energy associated with a nuclear reaction is
A)the total change in rest energy as a result of the reaction.
B)equivalent to the disintegration energy.
C)the minimum energy necessary for such a reaction to occur.
D)called the threshold energy.
E)the binding energy of the nucleons.
A)the total change in rest energy as a result of the reaction.
B)equivalent to the disintegration energy.
C)the minimum energy necessary for such a reaction to occur.
D)called the threshold energy.
E)the binding energy of the nucleons.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
33
What is the disintegration energy (in MeV)associated with this spontaneous decay? 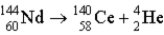
MNd = 143.910 083 u
MCe = 139.905 434 u
MHe = 4.002 603 u
1 u = 1.66 × 10−27 kg
A)1.54
B)2.37
C)1.90
D)4.13
E)8.21
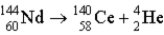
MNd = 143.910 083 u
MCe = 139.905 434 u
MHe = 4.002 603 u
1 u = 1.66 × 10−27 kg
A)1.54
B)2.37
C)1.90
D)4.13
E)8.21

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
34
The chart below shows part of the radioactive series beginning with the isotope 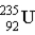
)The isotope marked with an X is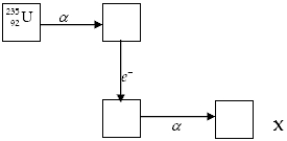
A)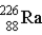 .
.
B)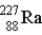 .
.
C)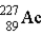 .
.
D)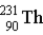 .
.
E)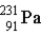 .
.
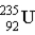
)The isotope marked with an X is

A)
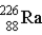 .
.B)
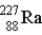 .
.C)
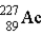 .
.D)
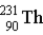 .
.E)
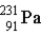 .
.
Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
35
Rutherford's experiment,in which he fired alpha particles of 7.7 MeV kinetic energy at a thin gold foil,showed that nuclei were very much smaller than the size of an atom because
A)some alpha particles passed through the foil undeflected.
B)some alpha particles were deflected backwards.
C)some alpha particles were captured by the gold nuclei.
D)the alpha particles could not get closer than 10−10 m to the gold nuclei.
E)the alpha particles split into deuterium nuclei when they encountered the gold nuclei.
A)some alpha particles passed through the foil undeflected.
B)some alpha particles were deflected backwards.
C)some alpha particles were captured by the gold nuclei.
D)the alpha particles could not get closer than 10−10 m to the gold nuclei.
E)the alpha particles split into deuterium nuclei when they encountered the gold nuclei.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
36
According to the shell model,binding energy per nucleon is greater when N or Z is equal to one of the numbers below except for
A)2.
B)8.
C)13.
D)20.
E)28.
A)2.
B)8.
C)13.
D)20.
E)28.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
37
When a neutron decays,a proton and an electron are observed.When the electrons emitted from a sample of neutrons are observed,they are found to have different kinetic energies.This was accounted for by
A)introducing a different particle,the neutrino.
B)introducing the effect of gravity on the particles.
C)including the kinetic energies of the neutron and proton.
D)modifying the laws of conservation of momentum and energy.
E)taking into account the uncertainties associated with Heisenberg's Uncertainty Principle.
A)introducing a different particle,the neutrino.
B)introducing the effect of gravity on the particles.
C)including the kinetic energies of the neutron and proton.
D)modifying the laws of conservation of momentum and energy.
E)taking into account the uncertainties associated with Heisenberg's Uncertainty Principle.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
38
In nuclear magnetic resonance,nuclei absorb energy when flipping between nuclear
A)mass states.
B)spin states.
C)charge states.
D)decay states.
E)isotope states.
A)mass states.
B)spin states.
C)charge states.
D)decay states.
E)isotope states.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
39
Two nuclei which share the same mass number A always are (Hint: Eliminate any wrong answers. )
A)stable.
B)unstable.
C)isotopes.
D)isobars.
E)radioactive.
A)stable.
B)unstable.
C)isotopes.
D)isobars.
E)radioactive.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
40
Heavy nuclei are unstable because
A)each nucleon is a separate particle that is not acted on by the nuclear force.
B)there are not enough protons present relative to the number of neutrons for the electrical force to be strong enough.
C)the nuclear force dominates the Coulomb repulsive force at distances less than 2 fm,but falls off rapidly at greater distances.
D)nuclei are stable only when the number of neutrons equals the number of protons.
E)nuclei are stable only when the number of protons exceeds the number of neutrons.
A)each nucleon is a separate particle that is not acted on by the nuclear force.
B)there are not enough protons present relative to the number of neutrons for the electrical force to be strong enough.
C)the nuclear force dominates the Coulomb repulsive force at distances less than 2 fm,but falls off rapidly at greater distances.
D)nuclei are stable only when the number of neutrons equals the number of protons.
E)nuclei are stable only when the number of protons exceeds the number of neutrons.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
41
The mass of 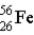
is 55.9349 u and the mass of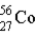
is 55.939 9 u.Which isobar decays into the other,and by what 2 possible processes?
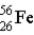
is 55.9349 u and the mass of
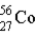
is 55.939 9 u.Which isobar decays into the other,and by what 2 possible processes?

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
42
In which of the following decays does the atomic mass number of the daughter nucleus differ from that of the parent nucleus?
A)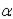
B)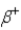
C)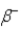
D)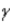
E)Answers (a), (b),and (c)are correct.
A)
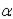
B)
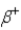
C)
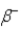
D)
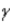
E)Answers (a), (b),and (c)are correct.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
43
A glass container holds equal numbers of atoms of phosphorus 30 with a half-life of 2.5 minutes and of nitrogen 13 with a half-life of 10 minutes.After 20 minutes the ratio of the number of nitrogen atoms remaining to the number of phosphorus atoms remaining is
A)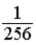 .
.
B)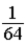 .
.
C)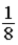 .
.
D)64.
E)256.
A)
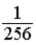 .
.B)
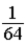 .
.C)
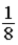 .
.D)64.
E)256.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
44
The radiocarbon content of 14C decreases after the death of a living system with a half-life of 5730 y.If an archaeologist working a dig finds an ancient firepit containing some partially consumed firewood and the wood contains only 12.5 percent of the 14C content of an equal carbon sample from a present-day tree,what is the age of the ancient site?

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
45
One of the naturally occurring radioactive series begins with 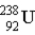
)Which of the following isotopes is the stable isotope at the end of this series?
A)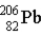
B)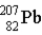
C)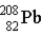
D)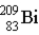
E)None of the above choices can be correct.
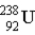
)Which of the following isotopes is the stable isotope at the end of this series?
A)
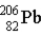
B)
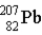
C)
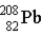
D)
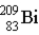
E)None of the above choices can be correct.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
46
Homer says that we can safely use nuclear power because all radioactive nuclei are gone after two half-lives.Marge says that only the decay rate is zero after two half-lives.Which one,if either,is correct,and why?
A)Homer,because half of the nuclei disintegrate in each half-life.
B)Marge,because the number of decays per unit time is halved in each half-life.
C)Homer,because it's safe to handle radioactive substances after two half-lives.
D)Both,because when all nuclei disintegrate the decay rate is also zero.
E)Neither,because one quarter of the nuclei are left after two half-lives.
A)Homer,because half of the nuclei disintegrate in each half-life.
B)Marge,because the number of decays per unit time is halved in each half-life.
C)Homer,because it's safe to handle radioactive substances after two half-lives.
D)Both,because when all nuclei disintegrate the decay rate is also zero.
E)Neither,because one quarter of the nuclei are left after two half-lives.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
47
The half-life of 131I is 8 days.On a certain day,the activity of an 131I sample is 6.4 mCi.What is its activity 40 days later?

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
48
The chart below shows part of the radioactive series beginning with the isotope 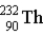
)The isotope marked with an X is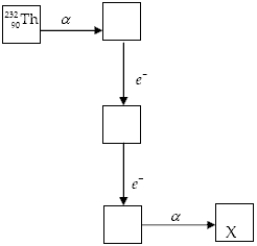
A)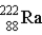 .
.
B)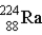 .
.
C)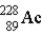 .
.
D)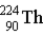 .
.
E)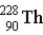 .
.
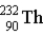
)The isotope marked with an X is

A)
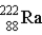 .
.B)
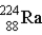 .
.C)
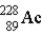 .
.D)
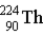 .
.E)
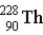 .
.
Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
49
Linus claims that the added gravitational force of neutrons holds the particles in a nucleus together.Linnea says that they stick together because they lose their electric charge when they form a nucleus.Which one,if either,is correct,and why?
A)Linus,because more particles exert gravitational forces on one another than exert electromagnetic forces.
B)Linus,because the numerical magnitude of G/ke is 7.42 × 10−21.
C)Linnea,because the numerical magnitude of G/ke is 7.42 × 10−21.
D)Both,because electric charge is lost and then gravity holds the nucleus together.
E)Neither,because gravity is not lost,and the numerical magnitude of ke/G is 1.35 × 1020.
A)Linus,because more particles exert gravitational forces on one another than exert electromagnetic forces.
B)Linus,because the numerical magnitude of G/ke is 7.42 × 10−21.
C)Linnea,because the numerical magnitude of G/ke is 7.42 × 10−21.
D)Both,because electric charge is lost and then gravity holds the nucleus together.
E)Neither,because gravity is not lost,and the numerical magnitude of ke/G is 1.35 × 1020.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
50
A pure sample of 226Ra contains 2.0 × 1014 atoms of the isotope.If the half-life of 226Ra = 1.6 × 103 years,what is the decay rate of this sample? (1 Ci = 3.7 × 1010 decays/s)

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck


