Exam 44: Nuclear Structure
Exam 1: Physics and Measurement25 Questions
Exam 2: Motion in One Dimension66 Questions
Exam 3: Vectors47 Questions
Exam 4: Motion in Two Dimensions79 Questions
Exam 5: The Laws of Motion113 Questions
Exam 6: Circular Motion and Other Applications of Newtons Laws55 Questions
Exam 7: Energy of a System74 Questions
Exam 8: Conservation of Energy84 Questions
Exam 9: Linear Momentum and Collisions89 Questions
Exam 10: Rotation of a Rigid Object About a Fixed Axis82 Questions
Exam 11: Angular Momentum46 Questions
Exam 12: Static Equilibrium and Elasticity34 Questions
Exam 13: Universal Gravitation47 Questions
Exam 14: Fluid Mechanics53 Questions
Exam 15: Oscillatory Motion41 Questions
Exam 16: Wave Motion46 Questions
Exam 17: Sound Waves48 Questions
Exam 18: Superposition and Standing Waves60 Questions
Exam 19: Temperature47 Questions
Exam 20: The First Law of Thermodynamics61 Questions
Exam 21: The Kinetic Theory of Gases38 Questions
Exam 22: Heat Engines, entropy, and the Second Law of Thermodynamics55 Questions
Exam 23: Electric Fields83 Questions
Exam 24: Gausss Law80 Questions
Exam 25: Electric Potential97 Questions
Exam 26: Capacitance and Dielectrics63 Questions
Exam 27: Current and Resistance34 Questions
Exam 28: Direct-Current Circuits84 Questions
Exam 29: Magnetic Fields80 Questions
Exam 30: Sources of the Magnetic Field105 Questions
Exam 31: Faradays Law62 Questions
Exam 32: Inductance23 Questions
Exam 33: Alternating-Current Circuits65 Questions
Exam 34: Electromagnetic Waves40 Questions
Exam 35: The Nature of Light and the Principles of Ray Optics38 Questions
Exam 36: Image Formation46 Questions
Exam 37: Wave Optics48 Questions
Exam 38: Diffraction Patterns and Polarization47 Questions
Exam 39: Relativity34 Questions
Exam 40: Introduction to Quantum Physics48 Questions
Exam 41: Quantum Mechanics33 Questions
Exam 42: Atomic Physics59 Questions
Exam 43: Molecules and Solids46 Questions
Exam 44: Nuclear Structure50 Questions
Exam 45: Applications of Nuclear Physics40 Questions
Exam 46: Particle Physics and Cosmology34 Questions
Select questions type
In which of the following decays does the atomic mass number of the daughter nucleus differ from that of the parent nucleus?
Free
(Multiple Choice)
5.0/5  (31)
(31)
Correct Answer:
A
Calculate the binding energy per nucleon (MeV/nucleon)for tritium, ( 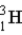 )a radioactive isotope of hydrogen.
Assume:
M p = 1.007 825 u
M n = 1.008 665 u
M t = 3.016 05 u
U = 1.66 × 10−27 kg
)a radioactive isotope of hydrogen.
Assume:
M p = 1.007 825 u
M n = 1.008 665 u
M t = 3.016 05 u
U = 1.66 × 10−27 kg
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
A
The chart below shows part of the radioactive series beginning with the isotope 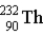 )The isotope marked with an X is
)The isotope marked with an X is 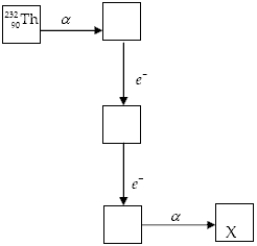
Free
(Multiple Choice)
4.7/5  (46)
(46)
Correct Answer:
B
Two nuclei may have equal Z,but different A,because they contain
(Multiple Choice)
4.9/5  (33)
(33)
Homer says that we can safely use nuclear power because all radioactive nuclei are gone after two half-lives.Marge says that only the decay rate is zero after two half-lives.Which one,if either,is correct,and why?
(Multiple Choice)
4.9/5  (29)
(29)
Find the ratio of the binding energy per nucleon for helium ( 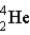 )to uranium-238 (
)to uranium-238 ( 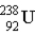 ))
Assume:
M p = 1.007 825 u
M n = 1.008 665 u
MHe = 4.002 603 u
MU = 238.050 786 u
U = 1.66 × 10−27 kg
))
Assume:
M p = 1.007 825 u
M n = 1.008 665 u
MHe = 4.002 603 u
MU = 238.050 786 u
U = 1.66 × 10−27 kg
(Multiple Choice)
4.8/5  (37)
(37)
What value of Z (atomic number)and A (mass number)result in the following β-decay? 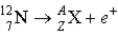
(Multiple Choice)
4.7/5  (34)
(34)
For large mass number nuclei which are stable,the ratio of protons to neutrons is
(Multiple Choice)
4.7/5  (31)
(31)
What value of Z (atomic number)and A (mass number)result in the following β-decay? 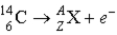
(Multiple Choice)
4.7/5  (36)
(36)
A pure sample of 226Ra contains 2.0 × 1014 atoms of the isotope.If the half-life of 226Ra = 1.6 × 103 years,what is the decay rate of this sample? (1 Ci = 3.7 × 1010 decays/s)
(Short Answer)
4.9/5  (35)
(35)
What value of Z (atomic number)and A (mass number)result in the following gamma decay? 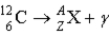
(Multiple Choice)
4.9/5  (33)
(33)
Because we know that the half-lives of many radioactive isotopes are millions of years,we can deduce that
(Multiple Choice)
4.8/5  (29)
(29)
The isotope,tritium,has a half-life of 12.3 years.Assume we have 10 kg of the substance.What will be the disintegration constant (in s−1)?
(Multiple Choice)
4.9/5  (35)
(35)
Two nuclei which share the same mass number A always are (Hint: Eliminate any wrong answers. )
(Multiple Choice)
4.7/5  (39)
(39)
Naturally radioactive nuclei can decay spontaneously by emitting the following particles:
(Multiple Choice)
4.8/5  (41)
(41)
Find the binding energy (in MeV)of carbon-12.
Assume:
MC = 12.000 000 u
M p = 1.007 825 u
M n = 1.008 665 u
U = 1.66 × 10−27 kg
(Multiple Choice)
4.9/5  (44)
(44)
What value of Z (atomic number)and A (mass number)result in the following alpha decay? 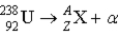
(Multiple Choice)
4.8/5  (27)
(27)
A glass container holds equal numbers of atoms of phosphorus 30 with a half-life of 2.5 minutes and of nitrogen 13 with a half-life of 10 minutes.After 20 minutes the ratio of the number of nitrogen atoms remaining to the number of phosphorus atoms remaining is
(Multiple Choice)
4.8/5  (30)
(30)
The ratio of the radius of a classical electron (re = kee2/mec2 = 2.8 × 10−15 m)to the radius of a 4He nucleus (r = r0A1/3)is
(Multiple Choice)
4.9/5  (37)
(37)
Showing 1 - 20 of 50
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)