Deck 15: How Atoms Bond and Molecules Attract
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/150
Play
Full screen (f)
Deck 15: How Atoms Bond and Molecules Attract
1
What is the valence shell?
A)It is the outermost shell of electrons in an atom.
B)It is the shell of electrons in an atom that is the least reactive.
C)It is the last partially filled orbital in an atom.
D)It is the shell of electrons in element V (atomic no. = 23)
E)It is the same as the orbital configuration.
A)It is the outermost shell of electrons in an atom.
B)It is the shell of electrons in an atom that is the least reactive.
C)It is the last partially filled orbital in an atom.
D)It is the shell of electrons in element V (atomic no. = 23)
E)It is the same as the orbital configuration.
A
2
How many valence electrons does gallium (Ga, atomic no. = 31)have?
A)1
B)6
C)3
D)31
E)70
A)1
B)6
C)3
D)31
E)70
C
3
What is one role of unpaired valance electrons?
A)They take part in the formation of different types of bonds.
B)They keep the paired electrons separated to minimize interaction.
C)They are the nonbonding electrons.
D)They provide the number of Lewis dots.
E)They tell us which Lewis dot structure is correct.
A)They take part in the formation of different types of bonds.
B)They keep the paired electrons separated to minimize interaction.
C)They are the nonbonding electrons.
D)They provide the number of Lewis dots.
E)They tell us which Lewis dot structure is correct.
A
4
An atom loses an electron to another atom. Is this an example of a physical or chemical change?
A)physical change involving the formation of negative ions
B)chemical change involving the formation of neutral atoms
C)physical change involving the formation of positive ions
D)chemical change involving the formation of ions
A)physical change involving the formation of negative ions
B)chemical change involving the formation of neutral atoms
C)physical change involving the formation of positive ions
D)chemical change involving the formation of ions

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
5
How many more electrons can fit within the valence shell of a hydrogen atom?
A)1
B)2
C)7
D)0
A)1
B)2
C)7
D)0

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
6
How do the electron-dot structures of elements in the same group in the periodic table compare with one another?
A)The structures differ by exactly two electrons between vertically consecutive elements.
B)The number of valence shell electrons increases by one for each element from the top to the bottom of the group.
C)Elements of the same group have the same number of valence electrons.
D)The number of electrons in the electron-dot-structure will equal the group number for each element of the group.
A)The structures differ by exactly two electrons between vertically consecutive elements.
B)The number of valence shell electrons increases by one for each element from the top to the bottom of the group.
C)Elements of the same group have the same number of valence electrons.
D)The number of electrons in the electron-dot-structure will equal the group number for each element of the group.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
7
How is it possible for a neutral molecule, such as water, to form an ion?
A)It can combine with a hydrogen ion to form a positively charged species.
B)It can combine with a chloride ion to form a negatively charged species.
C)It can fragment into protons and electrons.
D)It can absorb electrons and become negatively charged.
E)It can absorb electrons and become positively charged.
A)It can combine with a hydrogen ion to form a positively charged species.
B)It can combine with a chloride ion to form a negatively charged species.
C)It can fragment into protons and electrons.
D)It can absorb electrons and become negatively charged.
E)It can absorb electrons and become positively charged.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
8
How is the number of unpaired valence electrons in an atom related to the number of bonds that the atom can form?
A)There is no defined relationship between the number of unpaired valence electrons and number of bonds that the atom can form.
B)The number of unpaired valence electrons in an atom is one-half the number of bonds that the atom can form.
C)The number of unpaired valence electrons in an atom is twice the number of bonds that the atom can form.
D)The number of unpaired valence electrons in an atom is the same as the number of bonds that the atom can form.
A)There is no defined relationship between the number of unpaired valence electrons and number of bonds that the atom can form.
B)The number of unpaired valence electrons in an atom is one-half the number of bonds that the atom can form.
C)The number of unpaired valence electrons in an atom is twice the number of bonds that the atom can form.
D)The number of unpaired valence electrons in an atom is the same as the number of bonds that the atom can form.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
9
If carbonic acid (H2CO3)were to undergo ionization, what would one of the products be?
A)H-
B)CO2
C)H2O
D)CO3-1
E)CO3-2
A)H-
B)CO2
C)H2O
D)CO3-1
E)CO3-2

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
10
How many valence electrons does bromine (Br, atomic no. = 35)have?
A)1
B)7
C)21
D)28
E)35
A)1
B)7
C)21
D)28
E)35

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following elements has six valence electrons?
A)Be
B)B
C)C
D)N
E)O
A)Be
B)B
C)C
D)N
E)O

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is the correct electron dot structure for carbon (atomic no. = 6)? 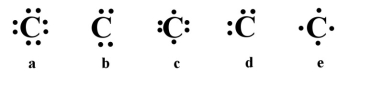
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
13
The concept of a chemical bond is ________.
A)how two or more atoms are held together
B)the sharing of nucleons
C)how two or more electrons reside in an orbital
D)how much energy it takes to remove an electron from a set of atoms
E)none of the above
A)how two or more atoms are held together
B)the sharing of nucleons
C)how two or more electrons reside in an orbital
D)how much energy it takes to remove an electron from a set of atoms
E)none of the above

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
14
Many of the macroscopic properties of a compound depend on ________.
A)how the atoms of the molecules are held together
B)the mass of the constituent atoms
C)the number of nucleons present in the sample
D)the size of the sample
E)how the atoms absorb light and the shape of the orbitals
A)how the atoms of the molecules are held together
B)the mass of the constituent atoms
C)the number of nucleons present in the sample
D)the size of the sample
E)how the atoms absorb light and the shape of the orbitals

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
15
How many valence electrons does boron (B, atomic no. = 5)have?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is the correct electron dot structure for chlorine (atomic no. = 17)? 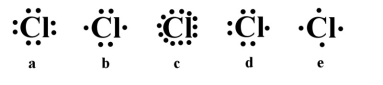
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following has the greatest number of nonbonding pairs of electrons ?
A)C
B)H
C)He
D)F
E)S
A)C
B)H
C)He
D)F
E)S

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
18
What is the name for the following polyatomic ion? CH3CO2-1
A)acetate
B)monocarboxylate
C)carboxylic
D)acidic
E)acetic
A)acetate
B)monocarboxylate
C)carboxylic
D)acidic
E)acetic

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
19
Why is it so easy for a magnesium atom to lose two electrons?
A)The nuclear charge of the magnesium atoms is relatively weak.
B)These two electrons are found relatively far from the nucleus.
C)These two electrons are well shielded from the nuclear charge.
D)There are lots of electron-electron repulsions that go on within the valence shell.
A)The nuclear charge of the magnesium atoms is relatively weak.
B)These two electrons are found relatively far from the nucleus.
C)These two electrons are well shielded from the nuclear charge.
D)There are lots of electron-electron repulsions that go on within the valence shell.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following elements has two valence electrons?
A)Na
B)Mg
C)H
D)Ne
E)Li
A)Na
B)Mg
C)H
D)Ne
E)Li

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
21
What needs to be done to convert a neutral nitrogen atom into an N-3 species?
A)add three electrons
B)remove three electrons
C)remove three protons
D)add three protons
E)add three nitrogens
A)add three electrons
B)remove three electrons
C)remove three protons
D)add three protons
E)add three nitrogens

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
22
Which should be larger, the potassium atom, K, or the potassium ion, K⁺?
A)The potassium ion, K⁺ is larger since charging an atom always makes it larger.
B)The potassium atom, K, with an additional shell of electrons is larger.
C)The potassium ion, K⁺ is larger since it has an extra electron which increases its size.
D)The potassium atom and the potassium ion are exactly the same size and only differ in charge.
A)The potassium ion, K⁺ is larger since charging an atom always makes it larger.
B)The potassium atom, K, with an additional shell of electrons is larger.
C)The potassium ion, K⁺ is larger since it has an extra electron which increases its size.
D)The potassium atom and the potassium ion are exactly the same size and only differ in charge.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
23
The neon atom tends NOT to gain any additional electrons because
A)its nuclear charge is not great enough.
B)that would result in a positive ion.
C)of the repulsions they would experience with electrons in the same shell.
D)there is no more room available in its outermost occupied shell.
A)its nuclear charge is not great enough.
B)that would result in a positive ion.
C)of the repulsions they would experience with electrons in the same shell.
D)there is no more room available in its outermost occupied shell.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
24
If a neutral atom gains two electrons, what is the electrical charge of the atom?
A)-1
B)+1
C)-2
D)+2
E)neutral
A)-1
B)+1
C)-2
D)+2
E)neutral

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following elements will most likely form an ion with a +1 charge?
A)Na
B)Mg
C)Al
D)Si
E)Cl
A)Na
B)Mg
C)Al
D)Si
E)Cl

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
26
Why doesn't the sodium atom gain seven electrons so that its third shell becomes the filled outermost shell?
A)It would be too difficult for another atom to lose seven electrons.
B)Only six additional electrons are required to fill the outermost shell of sodium.
C)In gaining seven more electrons, sodium's fourth outer shell becomes filled.
D)Sodium's nuclear charge is not strong enough to hold that many more electrons.
A)It would be too difficult for another atom to lose seven electrons.
B)Only six additional electrons are required to fill the outermost shell of sodium.
C)In gaining seven more electrons, sodium's fourth outer shell becomes filled.
D)Sodium's nuclear charge is not strong enough to hold that many more electrons.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following would be an ion with a double positive charge?
A)an Mg atom that gains two electrons
B)an Mg atom that gains one electron
C)an Mg atom that loses two electrons
D)an Mg atom that loses one electron
E)none of the above
A)an Mg atom that gains two electrons
B)an Mg atom that gains one electron
C)an Mg atom that loses two electrons
D)an Mg atom that loses one electron
E)none of the above

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following is a positive ion?
A)Na+1
B)Ca+2
C)Mg+2
D)Al+3
E)all of the above
A)Na+1
B)Ca+2
C)Mg+2
D)Al+3
E)all of the above

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following elements will most likely not form an ion at all?
A)Na
B)O
C)Ar
D)Mg
E)Br
A)Na
B)O
C)Ar
D)Mg
E)Br

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following elements will most likely form an ion with a -2 charge?
A)Na
B)S
C)Ne
D)Mg
E)Cl
A)Na
B)S
C)Ne
D)Mg
E)Cl

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following would be a negative ion with a single charge?
A)an atom with 11 protons and 12 electrons
B)an atom with 11 protons and 11 electrons
C)an atom with 12 protons and 11 electrons
D)an atom with 10 protons and 12 electrons
E)none of the above
A)an atom with 11 protons and 12 electrons
B)an atom with 11 protons and 11 electrons
C)an atom with 12 protons and 11 electrons
D)an atom with 10 protons and 12 electrons
E)none of the above

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
32
What is the name for the following polyatomic ion? PO4-3
A)phosphate
B)phosphorus oxide
C)phosphinate
D)trioxo phosphoride
E)potassium
A)phosphate
B)phosphorus oxide
C)phosphinate
D)trioxo phosphoride
E)potassium

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following elements will most likely form an ion with a +2 charge?
A)Na
B)Mg
C)Ne
D)Si
E)Cl
A)Na
B)Mg
C)Ne
D)Si
E)Cl

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following is NOT an ion?
A)H+1
B)Br-
C)O2
D)Mg+2
E)NO3-
A)H+1
B)Br-
C)O2
D)Mg+2
E)NO3-

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is an ion?
A)2 H2
B)Br2
C)HCl
D)Au + 3 Br2
E)none of the above
A)2 H2
B)Br2
C)HCl
D)Au + 3 Br2
E)none of the above

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is a negative ion?
A)Na+1
B)Na
C)O-2
D)O
E)all of the above
A)Na+1
B)Na
C)O-2
D)O
E)all of the above

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
37
If a neutral atom loses one electron, what is the electrical charge of the atom?
A)-1
B)+1
C)-2
D)+2
E)neutral
A)-1
B)+1
C)-2
D)+2
E)neutral

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following elements will most likely form an ion with a -1 charge?
A)Na
B)S
C)Ne
D)Mg
E)Cl
A)Na
B)S
C)Ne
D)Mg
E)Cl

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
39
Why does an atom with many valence electrons tend to gain electrons rather than lose any?
A)Atoms with many valence electrons tend to have relatively weak forces of attraction between the valence electrons and the nucleus. Therefore, the outer electrons are free to attract other electrons.
B)There is stability in numbers. Atoms with many valence electrons are always attracting new electrons.
C)The old adage that "he who has, gets" is also true in atomic structure. Atoms with many valence electrons can essentially overpower atoms with few valence electrons and attract additional electrons.
D)Atoms with many valence electrons tend to have relatively strong forces of attraction between the valence electrons and the nucleus. This makes it easy for them to gain additional electrons.
A)Atoms with many valence electrons tend to have relatively weak forces of attraction between the valence electrons and the nucleus. Therefore, the outer electrons are free to attract other electrons.
B)There is stability in numbers. Atoms with many valence electrons are always attracting new electrons.
C)The old adage that "he who has, gets" is also true in atomic structure. Atoms with many valence electrons can essentially overpower atoms with few valence electrons and attract additional electrons.
D)Atoms with many valence electrons tend to have relatively strong forces of attraction between the valence electrons and the nucleus. This makes it easy for them to gain additional electrons.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
40
The neon atom tends NOT to lose any electrons because
A)of its relatively strong effective nuclear charge.
B)that would result in a negative ion.
C)its electrons are paired together within the same orbitals.
D)the ionization energy is so high.
A)of its relatively strong effective nuclear charge.
B)that would result in a negative ion.
C)its electrons are paired together within the same orbitals.
D)the ionization energy is so high.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
41
Is an ionic compound an example of a chemical compound, or is a chemical compound an example of an ionic compound?
A)An chemical compound is an example of a ionic compound.
B)Neither is an example of the other.
C)Each is an example of the other.
D)An ionic compound is an example of a chemical compound.
A)An chemical compound is an example of a ionic compound.
B)Neither is an example of the other.
C)Each is an example of the other.
D)An ionic compound is an example of a chemical compound.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
42
If the following generic atom were to undergo ionization, what would be the charge of the most likely product? 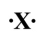
A)+2
B)-2
C)-6
D)+6
E)would probably not ionize
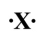
A)+2
B)-2
C)-6
D)+6
E)would probably not ionize

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
43
What is the compound that forms if you react potassium and sulfur?
A)K2S
B)KS
C)SP
D)PS2
E)SkP
A)K2S
B)KS
C)SP
D)PS2
E)SkP

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
44
How many oxide ions (O-2)are needed to balance the positive charge of a titanium ion (Ti+4)?
A)2
B)1
C)3
D)4
E)6
A)2
B)1
C)3
D)4
E)6

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following compounds contains ionic bonds?
A)CH4
B)K2O
C)Cl2
D)OF2
E)none of the above
A)CH4
B)K2O
C)Cl2
D)OF2
E)none of the above

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
46
Which are closer together: the two nuclei within potassium fluoride, KF, or the two nuclei within molecular fluorine, F2?
A)KF
B)F2
C)Both are the same. Any atom bonded to F will have the same inter-nuclear separation.
D)It makes little sense to compare two molecules which exist in different physical states. KF is a solid while F2 is a gas.
A)KF
B)F2
C)Both are the same. Any atom bonded to F will have the same inter-nuclear separation.
D)It makes little sense to compare two molecules which exist in different physical states. KF is a solid while F2 is a gas.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following substances contains F- ions?
A)PF3
B)F2
C)CF4
D)CaF2
E)all of the above
A)PF3
B)F2
C)CF4
D)CaF2
E)all of the above

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
48
Which would you expect to have a higher melting point: sodium chloride, NaCl, or aluminum oxide, Al2O3?
A)The aluminum oxide has a higher melting point because it is a larger molecule and has a greater number of molecular interactions.
B)NaCl has a higher melting point because it is a solid at room temperature.
C)The aluminum oxide has a higher melting point because of the greater charges of the ions, and hence the greater force of attractions between them.
D)The aluminum oxide has a higher melting point because of the covalent bonds within the molecule.
A)The aluminum oxide has a higher melting point because it is a larger molecule and has a greater number of molecular interactions.
B)NaCl has a higher melting point because it is a solid at room temperature.
C)The aluminum oxide has a higher melting point because of the greater charges of the ions, and hence the greater force of attractions between them.
D)The aluminum oxide has a higher melting point because of the covalent bonds within the molecule.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
49
Magnesium ions carry a 2+ charge, and chloride ions carry a 1- charge. What is the chemical formula for the ionic compound magnesium chloride?
A)MgCl
B)Mg2Cl
C)MgCl2
D)Mg2Cl2
A)MgCl
B)Mg2Cl
C)MgCl2
D)Mg2Cl2

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following best describes ionic bonding?
A)two atoms sharing a set of electrons
B)two atoms exchanging a set of electrons
C)one atom giving up some of its electrons to another atom
D)when two elements with same charge are held together by electrostatic forces
E)none of the above
A)two atoms sharing a set of electrons
B)two atoms exchanging a set of electrons
C)one atom giving up some of its electrons to another atom
D)when two elements with same charge are held together by electrostatic forces
E)none of the above

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
51
If the following generic atom were to undergo ionization, what would be the charge of most likely product? 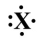
A)+3
B)-3
C)-5
D)8
E)would probably not ionize
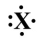
A)+3
B)-3
C)-5
D)8
E)would probably not ionize

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following molecules contains an ionic bond?
A)MgCl2
B)Cl2
C)SF3
D)PO4-3
E)none of the above
A)MgCl2
B)Cl2
C)SF3
D)PO4-3
E)none of the above

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following does not describe ionic compounds?
A)They have a tendency to melt easily.
B)They consist of positive and negative ions.
C)They are held together by electrostatic attraction.
D)They are usually very ordered.
E)none of the above
A)They have a tendency to melt easily.
B)They consist of positive and negative ions.
C)They are held together by electrostatic attraction.
D)They are usually very ordered.
E)none of the above

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
54
What molecule loses a proton to form the hydroxide ion, OH⁻?
A)The oxygen molecule, O2
B)The water molecule, H2O
C)The hydrogen peroxide molecule, H2O2
D)The hydrogen molecule, H2
A)The oxygen molecule, O2
B)The water molecule, H2O
C)The hydrogen peroxide molecule, H2O2
D)The hydrogen molecule, H2

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
55
If the following generic atom were to undergo ionization, what would the most likely product be? 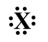
A)+1
B)-2
C)-3
D)+4
E)would probably not ionize
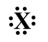
A)+1
B)-2
C)-3
D)+4
E)would probably not ionize

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
56
Take money away from your bank account and the bank will show a negative credit. Take an electron away from an atom, however, and the atom shows up positive. Explain.
A)Electrons are already negative. Therefore, we know from basic math that subtracting a negative (number)from a neutral (atom), will make the result positive.
B)Neutral atoms contain identically charged but oppositely signed protons and electrons. Removing one of the negative electrons results in an excess of positively charged protons.
C)Removing an electron from an atom does not have the atom show up positive. It simply leaves the atom short one electron.
D)Atoms are constantly exchanging electrons. Having an atom "show up positive" is only an expression indicating that it has taken its turn in the game of electron exchange.
A)Electrons are already negative. Therefore, we know from basic math that subtracting a negative (number)from a neutral (atom), will make the result positive.
B)Neutral atoms contain identically charged but oppositely signed protons and electrons. Removing one of the negative electrons results in an excess of positively charged protons.
C)Removing an electron from an atom does not have the atom show up positive. It simply leaves the atom short one electron.
D)Atoms are constantly exchanging electrons. Having an atom "show up positive" is only an expression indicating that it has taken its turn in the game of electron exchange.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
57
If you mix a typical iodine ion (I, atomic no. = 53)with a typical barium ion (Ba, atomic no. = 56), what compound is formed?
A)BaI2
B)BaI
C)Ba56I53
D)Ba2I
E)Ba2I2
A)BaI2
B)BaI
C)Ba56I53
D)Ba2I
E)Ba2I2

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
58
If you mix a typical aluminum ion (Al, atomic no. = 13)with a typical oxygen ion (O, atomic no. = 8), what compound is formed?
A)Al2O3
B)Al3O2
C)Al13O8
D)Al3O
E)Al2O2
A)Al2O3
B)Al3O2
C)Al13O8
D)Al3O
E)Al2O2

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
59
Barium ions carry a 2+ charge, and nitrogen ions carry a 3-charge. What would be the chemical formula for the ionic compound barium nitride?
A)Ba3N2
B)Ba2N3
C)Ba3N4
D)Ba2N2
A)Ba3N2
B)Ba2N3
C)Ba3N4
D)Ba2N2

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
60
How many chloride ions (Cl-1)are needed to balance the positive charge of a barium ion (Ba+2)?
A)2
B)1
C)-2
D)-1
E)3
A)2
B)1
C)-2
D)-1
E)3

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
61
What does the line in the following example actually represent? H-H
A)a shared pair of electrons
B)a covalent bond
C)an ionic bond
D)a pair of nonbonding electrons
E)A and B
A)a shared pair of electrons
B)a covalent bond
C)an ionic bond
D)a pair of nonbonding electrons
E)A and B

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
62
There is more gold in 1 km3 of the ocean than the amount of gold mined in all of recorded history. How come we do not mine the oceans?
A)It is too dilute to separate.
B)It would take too much energy.
C)It would cost too much.
D)all of the above
E)none of the above
A)It is too dilute to separate.
B)It would take too much energy.
C)It would cost too much.
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
63
Metals are useful for the structural support of buildings because they
A)do not conduct heat well
B)are shiny
C)conduct electricity
D)are strong but can be bent
E)c and d
A)do not conduct heat well
B)are shiny
C)conduct electricity
D)are strong but can be bent
E)c and d

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
64
How many covalent bonds would the following atom usually form? 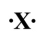
A)1
B)2
C)4
D)6
E)It would tend to form ionic bonds.
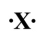
A)1
B)2
C)4
D)6
E)It would tend to form ionic bonds.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
65
Distinguish between a metal and a metal-containing compound.
A)There is no distinction between the two.
B)Only one of these contains ionic bonds.
C)Only one of these contains covalent bonds.
D)Only one of these occurs naturally.
A)There is no distinction between the two.
B)Only one of these contains ionic bonds.
C)Only one of these contains covalent bonds.
D)Only one of these occurs naturally.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
66
How many nonbonding pairs of electrons are in the following molecule? H-H
A)1 pair
B)6 pairs
C)0 pairs
D)8 pairs
E)none of the above
A)1 pair
B)6 pairs
C)0 pairs
D)8 pairs
E)none of the above

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
67
Metals are often used for making designer jewelry because they
A)do not conduct heat well
B)conduct electricity
C)are shiny
D)are strong but can be bent
E)c and d
A)do not conduct heat well
B)conduct electricity
C)are shiny
D)are strong but can be bent
E)c and d

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
68
What it the main difference between an ionic and a covalent bond?
A)One is the sharing of a pair of electrons, the other is the transfer of at least one electron.
B)One involves electrons, the other does not involve any electrons.
C)The electrons in both types of bonding undergo an exchange.
D)The electrons are traded between the two atoms and this keeps the atoms close.
E)Both bonds are the same, but named different to describe different atoms involved.
A)One is the sharing of a pair of electrons, the other is the transfer of at least one electron.
B)One involves electrons, the other does not involve any electrons.
C)The electrons in both types of bonding undergo an exchange.
D)The electrons are traded between the two atoms and this keeps the atoms close.
E)Both bonds are the same, but named different to describe different atoms involved.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
69
What property of metal atoms account for many of the observed bulk phenomena seen in metal samples?
A)Metal atoms easily lose one or more outer electrons.
B)Metal atoms easily gain one or more outer electrons.
C)Metals readily form ionic bonds.
D)Metals readily form covalent bonds.
E)none of the above
A)Metal atoms easily lose one or more outer electrons.
B)Metal atoms easily gain one or more outer electrons.
C)Metals readily form ionic bonds.
D)Metals readily form covalent bonds.
E)none of the above

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
70
Why is metal shiny?
A)The loose electrons reflect most wavelengths of light.
B)The electrons transmit most wavelengths of light.
C)The electrons absorb each light wave.
D)The electrons emit light due to electronic excitation.
E)all of the above
A)The loose electrons reflect most wavelengths of light.
B)The electrons transmit most wavelengths of light.
C)The electrons absorb each light wave.
D)The electrons emit light due to electronic excitation.
E)all of the above

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
71
If the concentration of gold in seawater is 2.0 milligram per ton of sea water and the mass of the ocean is 1.5 × 1018 tons, how much gold is in the ocean?
A)3.0 × 1012 kg
B)3.0 kg
C)300 g
D)36 mg
E)3,000 lb
A)3.0 × 1012 kg
B)3.0 kg
C)300 g
D)36 mg
E)3,000 lb

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
72
Why is it better to recycle metals than to mine more?
A)It takes far less energy to recycle.
B)Mining is less expensive than recycling but not environmentally friendly.
C)Ores contain toxic elements.
D)all of the above
E)none of the above
A)It takes far less energy to recycle.
B)Mining is less expensive than recycling but not environmentally friendly.
C)Ores contain toxic elements.
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
73
What is a molecule?
A)a group of atoms that are held together by covalent bonds
B)a group of atoms that are held together by ionic bonds
C)pair of atoms sharing a set of valence electrons
D)pair of shared valence electrons
E)group of covalent compounds held together by ionic bonds
A)a group of atoms that are held together by covalent bonds
B)a group of atoms that are held together by ionic bonds
C)pair of atoms sharing a set of valence electrons
D)pair of shared valence electrons
E)group of covalent compounds held together by ionic bonds

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
74
What property of alloys make them ideal for developing new materials?
A)The characteristics of the material change depending on how much of each component is present.
B)Alloys are very rigid and are extremely resistant to chemical decomposition.
C)The size of the atoms involved is directly related the electrical conductivity.
D)all of the above
E)none of the above
A)The characteristics of the material change depending on how much of each component is present.
B)Alloys are very rigid and are extremely resistant to chemical decomposition.
C)The size of the atoms involved is directly related the electrical conductivity.
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
75
Which would you expect to have a higher melting point: sodium chloride, NaCl, or cesium chloride, CsCl? Why?
A)The cesium chloride has a higher melting point because larger ions of the same charge are able to attract more ions of the opposite charge.
B)The cesium chloride has a higher melting point because its ions are smaller, which makes the charges more dense.
C)The sodium chloride has a higher melting point because of the greater charges of the ions, and hence the greater force of attractions between them.
D)The sodium chloride has a higher melting point because its ions are smaller, which allows oppositely charged ions to get closer.
A)The cesium chloride has a higher melting point because larger ions of the same charge are able to attract more ions of the opposite charge.
B)The cesium chloride has a higher melting point because its ions are smaller, which makes the charges more dense.
C)The sodium chloride has a higher melting point because of the greater charges of the ions, and hence the greater force of attractions between them.
D)The sodium chloride has a higher melting point because its ions are smaller, which allows oppositely charged ions to get closer.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
76
MgCl2 crystals are composed of
A)units of MgCl2 molecules held together by dipole interactions.
B)groups of Mg2+ ions and Cl2 molecules.
C)units composed of six Mg atoms and six Cl2 molecules.
D)a multitude of Mg2+ ions and![<strong>MgCl<sub>2</sub> crystals are composed of</strong> A)units of MgCl<sub>2</sub> molecules held together by dipole interactions. B)groups of Mg<sup>2+</sup> ions and Cl<sub>2</sub> molecules. C)units composed of six Mg atoms and six Cl<sub>2</sub> molecules. D)a multitude of Mg<sup>2+</sup> ions and ions grouped together in a three-dimensional array with a 1:2 ratio of Mg<sup>2+</sup> to . E)a two-dimensional array of [-Mg-Cl-Cl-] units.](https://storage.examlex.com/TB4071/11ea8004_e386_8611_846e_d1e53d034a2e_TB4071_11.jpg) ions grouped together in a three-dimensional array with a 1:2 ratio of Mg2+ to
ions grouped together in a three-dimensional array with a 1:2 ratio of Mg2+ to ![<strong>MgCl<sub>2</sub> crystals are composed of</strong> A)units of MgCl<sub>2</sub> molecules held together by dipole interactions. B)groups of Mg<sup>2+</sup> ions and Cl<sub>2</sub> molecules. C)units composed of six Mg atoms and six Cl<sub>2</sub> molecules. D)a multitude of Mg<sup>2+</sup> ions and ions grouped together in a three-dimensional array with a 1:2 ratio of Mg<sup>2+</sup> to . E)a two-dimensional array of [-Mg-Cl-Cl-] units.](https://storage.examlex.com/TB4071/11ea8004_e386_8612_846e_770bd8344801_TB4071_11.jpg) .
.
E)a two-dimensional array of [-Mg-Cl-Cl-] units.
A)units of MgCl2 molecules held together by dipole interactions.
B)groups of Mg2+ ions and Cl2 molecules.
C)units composed of six Mg atoms and six Cl2 molecules.
D)a multitude of Mg2+ ions and
![<strong>MgCl<sub>2</sub> crystals are composed of</strong> A)units of MgCl<sub>2</sub> molecules held together by dipole interactions. B)groups of Mg<sup>2+</sup> ions and Cl<sub>2</sub> molecules. C)units composed of six Mg atoms and six Cl<sub>2</sub> molecules. D)a multitude of Mg<sup>2+</sup> ions and ions grouped together in a three-dimensional array with a 1:2 ratio of Mg<sup>2+</sup> to . E)a two-dimensional array of [-Mg-Cl-Cl-] units.](https://storage.examlex.com/TB4071/11ea8004_e386_8611_846e_d1e53d034a2e_TB4071_11.jpg) ions grouped together in a three-dimensional array with a 1:2 ratio of Mg2+ to
ions grouped together in a three-dimensional array with a 1:2 ratio of Mg2+ to ![<strong>MgCl<sub>2</sub> crystals are composed of</strong> A)units of MgCl<sub>2</sub> molecules held together by dipole interactions. B)groups of Mg<sup>2+</sup> ions and Cl<sub>2</sub> molecules. C)units composed of six Mg atoms and six Cl<sub>2</sub> molecules. D)a multitude of Mg<sup>2+</sup> ions and ions grouped together in a three-dimensional array with a 1:2 ratio of Mg<sup>2+</sup> to . E)a two-dimensional array of [-Mg-Cl-Cl-] units.](https://storage.examlex.com/TB4071/11ea8004_e386_8612_846e_770bd8344801_TB4071_11.jpg) .
.E)a two-dimensional array of [-Mg-Cl-Cl-] units.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following is not a property of metal?
A)does not conduct heat well
B)conducts electricity
C)is shiny
D)is strong, but can be bent
E)All of the above are properties of metals.
A)does not conduct heat well
B)conducts electricity
C)is shiny
D)is strong, but can be bent
E)All of the above are properties of metals.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
78
Given that the total number of atoms on our planet remains fairly constant, how is it ever possible to deplete a natural resource such as a metal?
A)The problem remains that not everyone recycles as they should.
B)Recycling only forestalls the inevitable depletion of metal resources.
C)The atoms don't leave our planet, which is why naturally occurring materials never really reach the point of depletion.
D)The problem is with the expense of collecting metal atoms that are uniformly dispersed.
A)The problem remains that not everyone recycles as they should.
B)Recycling only forestalls the inevitable depletion of metal resources.
C)The atoms don't leave our planet, which is why naturally occurring materials never really reach the point of depletion.
D)The problem is with the expense of collecting metal atoms that are uniformly dispersed.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following describes how a metal atoms behaves in a bulk metallic object?
A)The metal ion shares its outermost electrons freely with its neighbors.
B)The metal atoms have limited interaction with neighboring atoms.
C)The metal atom shares its electrons in a very directional manner.
D)The metal atom shares its electrons with only one other atom.
E)none of the above
A)The metal ion shares its outermost electrons freely with its neighbors.
B)The metal atoms have limited interaction with neighboring atoms.
C)The metal atom shares its electrons in a very directional manner.
D)The metal atom shares its electrons with only one other atom.
E)none of the above

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
80
Why are ores so valuable?
A)They are sources of naturally occurring gold.
B)Metals can be efficiently extracted from them.
C)They tend to occur in scenic mountainous regions.
D)They hold many clues to Earth's natural history.
A)They are sources of naturally occurring gold.
B)Metals can be efficiently extracted from them.
C)They tend to occur in scenic mountainous regions.
D)They hold many clues to Earth's natural history.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck


