Exam 15: How Atoms Bond and Molecules Attract
Exam 1: Patterns of Motion and Equilibrium94 Questions
Exam 2: Newtons Laws of Motion109 Questions
Exam 3: Momentum and Energy128 Questions
Exam 4: Gravity, Projectiles, and Satellites114 Questions
Exam 5: Fluid Mechanics120 Questions
Exam 6: Thermal Energy and Thermodynamics100 Questions
Exam 7: Heat Transfer and Change of Phase115 Questions
Exam 8: Static and Current Electricity144 Questions
Exam 9: Magnetism and Electromagnetic Induction105 Questions
Exam 10: Waves and Sound120 Questions
Exam 11: Light146 Questions
Exam 12: Atoms and the Periodic Table128 Questions
Exam 13: The Atomic Nucleus and Radioactivity124 Questions
Exam 14: Elements of Chemistry49 Questions
Exam 15: How Atoms Bond and Molecules Attract150 Questions
Exam 16: Mixtures141 Questions
Exam 17: How Chemicals React118 Questions
Exam 18: Two Classes of Chemical Reactions182 Questions
Exam 19: Organic Compounds98 Questions
Exam 20: Rocks and Minerals170 Questions
Exam 21: Plate Tectonics and Earths Interior175 Questions
Exam 22: Shaping Earths Surface175 Questions
Exam 23: Geologic Timereading the Rock Record145 Questions
Exam 24: The Oceans, Atmosphere, and Climatic Effects172 Questions
Exam 25: Driving Forces of Weather145 Questions
Exam 26: The Solar System87 Questions
Exam 27: Stars and Galaxies84 Questions
Exam 28: The Structure of Space and Time55 Questions
Exam 29: Prologue: the Nature of Science22 Questions
Select questions type
The separation of charges within a polar molecule is called a(n)________.
Free
(Multiple Choice)
4.8/5  (42)
(42)
Correct Answer:
A
If the following generic atom were to undergo ionization, what would the most likely product be? 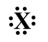
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
E
Which of the following elements will most likely form an ion with a +2 charge?
Free
(Multiple Choice)
4.9/5  (36)
(36)
Correct Answer:
B
Why does an atom with many valence electrons tend to gain electrons rather than lose any?
(Multiple Choice)
4.8/5  (40)
(40)
How many oxide ions (O-2)are needed to balance the positive charge of a titanium ion (Ti+4)?
(Multiple Choice)
4.8/5  (32)
(32)
Metals are useful for the structural support of buildings because they
(Multiple Choice)
4.9/5  (36)
(36)
Which would you expect to have a higher melting point: sodium chloride, NaCl, or aluminum oxide, Al2O3?
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following is the correct electron dot structure for carbon (atomic no. = 6)? 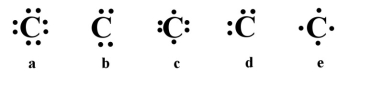
(Multiple Choice)
4.9/5  (39)
(39)
Consider the boiling points of the following compounds and their solubilities in room-temperature water. Why does the solubilities in water go down as the boiling points of these alcohols go up. 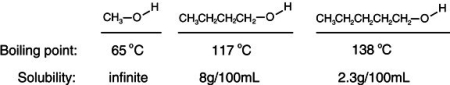
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following molecules has the highest boiling point?
(Multiple Choice)
4.7/5  (37)
(37)
An atom loses an electron to another atom. Is this an example of a physical or chemical change?
(Multiple Choice)
4.9/5  (31)
(31)
Which are closer together: the two nuclei within potassium fluoride, KF, or the two nuclei within molecular fluorine, F2?
(Multiple Choice)
5.0/5  (40)
(40)
Is an ionic compound an example of a chemical compound, or is a chemical compound an example of an ionic compound?
(Multiple Choice)
4.9/5  (33)
(33)
Atoms of metallic elements can form ionic bonds, but they are not very good at forming covalent bonds. Why?
(Multiple Choice)
4.8/5  (28)
(28)
Which should be larger, the potassium atom, K, or the potassium ion, K⁺?
(Multiple Choice)
5.0/5  (37)
(37)
Showing 1 - 20 of 150
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)