Deck 30: Nuclear Physics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/175
Play
Full screen (f)
Deck 30: Nuclear Physics
1
The symbol for a certain isotope of radium is 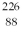 Ra. How many nucleons are there in the nucleus of this isotope?
Ra. How many nucleons are there in the nucleus of this isotope?
A) 88
B) 138
C) 214
D) 226
E) 314
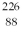 Ra. How many nucleons are there in the nucleus of this isotope?
Ra. How many nucleons are there in the nucleus of this isotope?A) 88
B) 138
C) 214
D) 226
E) 314
D
2
Name the first three isotopes of hydrogen, write their symbols in standard notation, and indicate which one is the most common and which one is the most unstable.
 H is ordinary hydrogen,
H is ordinary hydrogen,  H is deuterium, and
H is deuterium, and 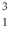 H is tritium.
H is tritium. 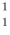 H is the most common and
H is the most common and 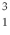 H is the most unstable.
H is the most unstable. 3
The primary reason that very large nuclei are unstable is due to
A) the cumulative repulsive force of the protons.
B) the cumulative attractive force between the protons and the orbiting electrons.
C) the repulsive force between the neutrons and the protons.
D) the extreme weakness of the gravitational attraction of the protons.
A) the cumulative repulsive force of the protons.
B) the cumulative attractive force between the protons and the orbiting electrons.
C) the repulsive force between the neutrons and the protons.
D) the extreme weakness of the gravitational attraction of the protons.
A
4
The atomic number and mass number for calcium-39 are 20 and 39, respectively. How many neutrons are in one atom?
A) 1
B) 19
C) 20
D) 39
E) 59
A) 1
B) 19
C) 20
D) 39
E) 59

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
5
The atomic number of an atom is equal to the number of what particles in the nucleus?
A) protons
B) neutrons
C) nucleons
D) electrons
E) positrons.
A) protons
B) neutrons
C) nucleons
D) electrons
E) positrons.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
6
What is true of an element of atomic number 15 that has an isotope of mass number 32? (There could be more than one correct choice.)
A) the number of protons in the nucleus is 15.
B) the number of neutrons in the nucleus is 15.
C) the number of protons in the nucleus is 32.
D) the number of nucleons in the nucleus is 47.
E) the number of neutrons in the nucleus is 17.
A) the number of protons in the nucleus is 15.
B) the number of neutrons in the nucleus is 15.
C) the number of protons in the nucleus is 32.
D) the number of nucleons in the nucleus is 47.
E) the number of neutrons in the nucleus is 17.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
7
The two strongest forces that act between protons in a nucleus are
A) the weak nuclear and electromagnetic forces.
B) the weak nuclear and the gravitational forces.
C) the electrostatic and the gravitational forces.
D) the strong nuclear and the electrostatic forces.
A) the weak nuclear and electromagnetic forces.
B) the weak nuclear and the gravitational forces.
C) the electrostatic and the gravitational forces.
D) the strong nuclear and the electrostatic forces.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
8
An yttrium isotope has 39 protons and 50 neutrons in its nucleus. Which one of the following symbols accurately represents this isotope?
A)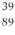 Y
Y
B)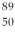 Y
Y
C)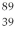 Y
Y
D)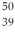 Y
Y
E)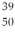 Y
Y
A)
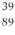 Y
YB)
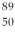 Y
YC)
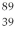 Y
YD)
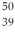 Y
YE)
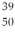 Y
Y
Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
9
The main reason that there is a limit to the size of a stable nucleus is
A) the limited range of the gravitational force.
B) the short range nature of the strong nuclear force.
C) the weakness of the gravitational force.
D) the weakness of the electrostatic force.
A) the limited range of the gravitational force.
B) the short range nature of the strong nuclear force.
C) the weakness of the gravitational force.
D) the weakness of the electrostatic force.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
10
The atomic mass unit is defined as
A) the mass of a proton.
B) the mass of an electron.
C) the mass of a hydrogen-1 atom.
D) one twelfth the mass of a carbon-12 atom.
E) the mass of a carbon-12 nucleus.
A) the mass of a proton.
B) the mass of an electron.
C) the mass of a hydrogen-1 atom.
D) one twelfth the mass of a carbon-12 atom.
E) the mass of a carbon-12 nucleus.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
11
Atomic nuclei that are all isotopes of an element all have the same
A) number of nucleons.
B) mass.
C) number of protons.
D) number of neutrons.
A) number of nucleons.
B) mass.
C) number of protons.
D) number of neutrons.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
12
The symbol for a certain isotope of strontium is 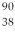 Sr. What is the mass number of this isotope?
Sr. What is the mass number of this isotope?
A) 38
B) 52
C) 88
D) 90
E) 128
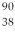 Sr. What is the mass number of this isotope?
Sr. What is the mass number of this isotope?A) 38
B) 52
C) 88
D) 90
E) 128

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
13
In massive stars, three helium nuclei fuse together, forming a carbon nucleus, and this reaction heats the core of the star. The net mass of the three helium nuclei must therefore be
A) higher than that of the carbon nucleus.
B) less than that of the carbon nucleus.
C) the same as that of the carbon nucleus because mass is always conserved.
D) the same as that of the carbon nucleus because energy is always conserved.
A) higher than that of the carbon nucleus.
B) less than that of the carbon nucleus.
C) the same as that of the carbon nucleus because mass is always conserved.
D) the same as that of the carbon nucleus because energy is always conserved.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following statements is not true of the strong nuclear force?
A) The nuclear force has a short range, of the order of nuclear dimensions.
B) For two protons that are very close together, the nuclear force and the electric force have about the same magnitudes.
C) The nuclear force does not depend on charge.
D) A nucleon in a large nucleus interacts via the nuclear force only with nearby nucleons, not with ones far away in the nucleus.
E) The nuclear force affects both neutrons and protons.
A) The nuclear force has a short range, of the order of nuclear dimensions.
B) For two protons that are very close together, the nuclear force and the electric force have about the same magnitudes.
C) The nuclear force does not depend on charge.
D) A nucleon in a large nucleus interacts via the nuclear force only with nearby nucleons, not with ones far away in the nucleus.
E) The nuclear force affects both neutrons and protons.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
15
Write the standard nuclear notation for the following nuclei: hydrogen-2, sulfur-33, and lead-207.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
16
In a 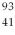 Nb nucleus, how many protons, neutrons, and electrons are found there?
Nb nucleus, how many protons, neutrons, and electrons are found there?
A) 41, 52, 93.
B) 41, 52, 41.
C) 52, 41, 41.
D) 41, 52, 0.
E) 93, 41, 93.
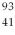 Nb nucleus, how many protons, neutrons, and electrons are found there?
Nb nucleus, how many protons, neutrons, and electrons are found there?A) 41, 52, 93.
B) 41, 52, 41.
C) 52, 41, 41.
D) 41, 52, 0.
E) 93, 41, 93.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
17
All nuclei of a given element have the same number of
A) neutrons.
B) protons.
C) nucleons.
D) protons + neutrons.
A) neutrons.
B) protons.
C) nucleons.
D) protons + neutrons.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
18
The symbol for a certain isotope of polonium is 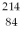 Po. How many neutrons are there in the nucleus of this isotope?
Po. How many neutrons are there in the nucleus of this isotope?
A) 84
B) 130
C) 214
D) 298
E) 314
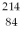 Po. How many neutrons are there in the nucleus of this isotope?
Po. How many neutrons are there in the nucleus of this isotope?A) 84
B) 130
C) 214
D) 298
E) 314

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
19
A stable nucleus contains many protons very close to each other, all positively charged. Why do the protons not fly apart due to mutual Coulomb repulsion?
A) An attractive nuclear force in the nucleus counteracts the effect of the Coulomb forces.
B) There are an equal number of electrons in the nucleus which neutralize the protons.
C) The neutrons in the nucleus shield the protons from each other.
D) The Coulomb force does not operate within nuclei.
E) The gravity of the protons and neutrons overcomes their repulsion at such close distances.
A) An attractive nuclear force in the nucleus counteracts the effect of the Coulomb forces.
B) There are an equal number of electrons in the nucleus which neutralize the protons.
C) The neutrons in the nucleus shield the protons from each other.
D) The Coulomb force does not operate within nuclei.
E) The gravity of the protons and neutrons overcomes their repulsion at such close distances.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
20
The mass number of an atom is equal to the number of what particles in the nucleus?
A) protons
B) neutrons
C) nucleons
D) electrons
E) positrons.
A) protons
B) neutrons
C) nucleons
D) electrons
E) positrons.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
21
What are the mass number, the atomic number, and neutron number for each of the following particles?
(a) proton
(b) neutron
(c) alpha particle
(a) proton
(b) neutron
(c) alpha particle

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
22
What are the mass number A and the charge (in units of e) for each of the following particles or rays?
(a) beta-plus
(b) beta-minus
(c) gamma ray
(a) beta-plus
(b) beta-minus
(c) gamma ray

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
23
In beta-minus decay
A) a proton is emitted.
B) a neutron is emitted.
C) an electron is emitted.
D) an electron decays into another particle.
E) a proton is transformed into a neutron.
A) a proton is emitted.
B) a neutron is emitted.
C) an electron is emitted.
D) an electron decays into another particle.
E) a proton is transformed into a neutron.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
24
What is the mass number of alpha particles?
A) 1
B) 2
C) 3
D) 4
E) 6
A) 1
B) 2
C) 3
D) 4
E) 6

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
25
A β- particle is also known as
A) an electron.
B) a positron.
C) a helium nucleus.
D) a high-energy photon.
A) an electron.
B) a positron.
C) a helium nucleus.
D) a high-energy photon.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
26
In β- decay, the number of protons in the nucleus is
A) decreased by 1.
B) decreased by 2.
C) increased by 1.
D) increased by 2.
E) remains unchanged.
A) decreased by 1.
B) decreased by 2.
C) increased by 1.
D) increased by 2.
E) remains unchanged.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
27
A β+ particle is also known as
A) an electron.
B) a positron.
C) a helium nucleus.
D) a high-energy photon.
A) an electron.
B) a positron.
C) a helium nucleus.
D) a high-energy photon.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
28
A fusion reaction releases energy because the binding energy of the resulting nucleus
A) is greater than the binding energy of the original nuclei.
B) is equal to the binding energy of the original nuclei.
C) is less than the binding energy of the original nuclei.
D) is released in the process.
E) is absorbed in the process.
A) is greater than the binding energy of the original nuclei.
B) is equal to the binding energy of the original nuclei.
C) is less than the binding energy of the original nuclei.
D) is released in the process.
E) is absorbed in the process.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
29
When a β- particle is emitted from an unstable nucleus, the atomic number of the nucleus
A) increases by 1.
B) decreases by 1.
C) increases by 2.
D) decreases by 2.
E) does not change.
A) increases by 1.
B) decreases by 1.
C) increases by 2.
D) decreases by 2.
E) does not change.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
30
During β+ decay
A) a neutron is transformed to a proton.
B) a proton is transformed to a neutron.
C) a neutron is ejected from the nucleus.
D) a proton is ejected from the nucleus.
E) the number of nucleons decreases.
A) a neutron is transformed to a proton.
B) a proton is transformed to a neutron.
C) a neutron is ejected from the nucleus.
D) a proton is ejected from the nucleus.
E) the number of nucleons decreases.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
31
In β- decay, the number of neutrons in the nucleus is
A) decreased by 1.
B) decreased by 2.
C) increased by 1.
D) increased by 2.
E) remains unchanged.
A) decreased by 1.
B) decreased by 2.
C) increased by 1.
D) increased by 2.
E) remains unchanged.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
32
In positron decay, the number of protons in the nucleus is
A) decreased by 1.
B) decreased by 2.
C) increased by 1.
D) increased by 2.
E) remains unchanged.
A) decreased by 1.
B) decreased by 2.
C) increased by 1.
D) increased by 2.
E) remains unchanged.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
33
When a β- particle is emitted from an unstable nucleus, the atomic mass number of the nucleus
A) increases by 1.
B) decreases by 1.
C) increases by 2.
D) does not change.
A) increases by 1.
B) decreases by 1.
C) increases by 2.
D) does not change.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
34
An α particle is also known as
A) an electron.
B) a positron.
C) a helium nucleus.
D) a high-energy photon.
A) an electron.
B) a positron.
C) a helium nucleus.
D) a high-energy photon.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
35
When a β+ particle is emitted from an unstable nucleus, the atomic number of the nucleus
A) increases by 1.
B) decreases by 1.
C) increases by 2.
D) decreases by 2.
E) does not change.
A) increases by 1.
B) decreases by 1.
C) increases by 2.
D) decreases by 2.
E) does not change.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
36
The nuclei of 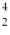 He are also known as
He are also known as
A) α particles.
B) β particles.
C) γ rays.
D) x-rays.
E) positrons.
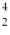 He are also known as
He are also known asA) α particles.
B) β particles.
C) γ rays.
D) x-rays.
E) positrons.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
37
The binding energy per nucleon of a nucleus
A) increases steadily as we go to heavier elements.
B) decreases steadily as we go to heavier elements.
C) is approximately constant throughout the periodic table, except for very light nuclei.
D) has a maximum near iron in the periodic table and decreases after that for heavier elements.
E) has a minimum near iron in the periodic table and increases after that for heavier elements.
A) increases steadily as we go to heavier elements.
B) decreases steadily as we go to heavier elements.
C) is approximately constant throughout the periodic table, except for very light nuclei.
D) has a maximum near iron in the periodic table and decreases after that for heavier elements.
E) has a minimum near iron in the periodic table and increases after that for heavier elements.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
38
A radioactive isotope of atomic number Z emits an alpha particle, and the daughter nucleus then emits a beta-minus particle. What is the atomic number of the resulting nucleus?
A) Z -1
B) Z + 1
C) Z - 2
D) Z - 3
A) Z -1
B) Z + 1
C) Z - 2
D) Z - 3

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
39
During β- decay
A) a neutron is transformed to a proton.
B) a proton is transformed to a neutron.
C) a neutron is ejected from the nucleus.
D) a proton is ejected from the nucleus.
E) the number of nucleons increases.
A) a neutron is transformed to a proton.
B) a proton is transformed to a neutron.
C) a neutron is ejected from the nucleus.
D) a proton is ejected from the nucleus.
E) the number of nucleons increases.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
40
A gamma ray is also known as
A) an electron.
B) a positron.
C) a helium nucleus.
D) a high-energy photon.
A) an electron.
B) a positron.
C) a helium nucleus.
D) a high-energy photon.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
41
The half-life of cobalt-60 is 5.3 years, while that of strontium-90 is about 29 years. Suppose you have samples of both isotopes, and that they initially have the same activity (number of decays per second). What must be true of the numbers of cobalt-60 and strontium-90 nuclei in these samples?
A) There are more strontium-90 than cobalt-60 nuclei.
B) There are equal numbers of cobalt-60 and strontium-90 nuclei.
C) There are more cobalt-60 than strontium-90 nuclei.
D) It is not possible to compare numbers of nuclei without knowing the masses of the samples.
A) There are more strontium-90 than cobalt-60 nuclei.
B) There are equal numbers of cobalt-60 and strontium-90 nuclei.
C) There are more cobalt-60 than strontium-90 nuclei.
D) It is not possible to compare numbers of nuclei without knowing the masses of the samples.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
42
The chief hazard of radiation damage to living cells is
A) due to heating.
B) due to ionization.
C) due to the creation of chemical impurities.
D) the creation of new isotopes within the body.
E) the creation of radioactive material within the body.
A) due to heating.
B) due to ionization.
C) due to the creation of chemical impurities.
D) the creation of new isotopes within the body.
E) the creation of radioactive material within the body.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
43
Suppose the half-life of an isotope is 2 days. You purchase 10 grams of the isotope, but it was produced in a laboratory 4 days before it was delivered to you. How much of this isotope will you have 3 days after it was delivered to you?
A) more than 2.5 grams but less than 5 grams
B) 2.5 grams
C) more than 1.25 grams but less than 2.5 grams
D) 1.25 grams
E) less than 1.25 grams
A) more than 2.5 grams but less than 5 grams
B) 2.5 grams
C) more than 1.25 grams but less than 2.5 grams
D) 1.25 grams
E) less than 1.25 grams

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
44
The half-life of cobalt-60 is 5.3 years, while that of strontium-90 is about 29 years. Suppose you have samples of both isotopes, and that they initially contain equal numbers of atoms of these isotopes. How will the activities (number of decays per second) of the samples compare?
A) The activity of the cobalt-60 sample will be greater.
B) The activities cannot be compared without more information.
C) The activities will be equal.
D) The activity of the strontium-90 sample will be greater.
A) The activity of the cobalt-60 sample will be greater.
B) The activities cannot be compared without more information.
C) The activities will be equal.
D) The activity of the strontium-90 sample will be greater.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
45
A β- decay occurs in an unstable nucleus when
A) a proton is converted to an electron by the strong force.
B) a proton is converted to a neutron by the strong force.
C) a neutron is converted to a proton by the weak force.
D) a neutron is converted to an alpha particle by the weak force.
E) a neutron is converted to a positron by the weak force.
A) a proton is converted to an electron by the strong force.
B) a proton is converted to a neutron by the strong force.
C) a neutron is converted to a proton by the weak force.
D) a neutron is converted to an alpha particle by the weak force.
E) a neutron is converted to a positron by the weak force.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
46
Two radioactive isotopes, X and Y, both decay to stable products. The half-life of X is about a day, while that of Y is about a week. Suppose a radioactive sample consists of a mixture of these two nuclides. If the mixture is such that the activities arising from X and Y are initially equal, then a few days later the activity of the sample will be due
A) predominantly to Y.
B) predominantly to X.
C) entirely to Y.
D) to X and Y equally.
A) predominantly to Y.
B) predominantly to X.
C) entirely to Y.
D) to X and Y equally.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
47
Carbon-14 decays by β- emission. What nucleus is left after this decay?
A) carbon-13
B) carbon-14
C) carbon-15
D) nitrogen-14
E) nitrogen-15
A) carbon-13
B) carbon-14
C) carbon-15
D) nitrogen-14
E) nitrogen-15

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
48
The atom 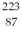 Fr decays to
Fr decays to 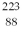 Ra by emitting what kind of nuclear radiation?
Ra by emitting what kind of nuclear radiation?
A) alpha
B) beta-minus
C) beta-plus
D) gamma
E) x-rays.
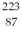 Fr decays to
Fr decays to 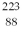 Ra by emitting what kind of nuclear radiation?
Ra by emitting what kind of nuclear radiation?A) alpha
B) beta-minus
C) beta-plus
D) gamma
E) x-rays.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
49
Modern in-air nuclear bomb tests have created an extra high level of 14C in our atmosphere. If future archaeologists date samples from this era, without knowing of this testing, will their carbon-14 dates be too young, too old, or correct? If correct, why?
A) Too young.
B) Too old.
C) Correct, since 14C from bomb tests is different from that produced naturally.
D) Correct, because modern biological materials do not gather 14C from bomb tests.
A) Too young.
B) Too old.
C) Correct, since 14C from bomb tests is different from that produced naturally.
D) Correct, because modern biological materials do not gather 14C from bomb tests.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
50
A person receives an absorbed dose of protons of 20 millirads. The RBE of protons is 5. What is this person's equivalent dose in rem?
A) 4 millirem
B) 15 millirem
C) 20 millirem
D) 25 millirem
E) 100 millirem
A) 4 millirem
B) 15 millirem
C) 20 millirem
D) 25 millirem
E) 100 millirem

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
51
What happens to the half-life of a radioactive substance as it decays?
A) It remains constant.
B) It increases.
C) It decreases at a constant rate.
D) It decreases at an exponential rate.
A) It remains constant.
B) It increases.
C) It decreases at a constant rate.
D) It decreases at an exponential rate.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
52
Polonium-216 decays to lead-212 by emitting what kind of nuclear radiation?
A) alpha
B) beta-minus
C) beta-plus
D) gamma
E) x-rays.
A) alpha
B) beta-minus
C) beta-plus
D) gamma
E) x-rays.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
53
How many protons, neutrons, and nucleons are there in a carbon-14 nucleus?

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
54
When an unstable nucleus decays by emitting an alpha particle, the atomic mass number A of the nucleus
A) increases by 4.
B) increases by 2.
C) decreases by 2.
D) decreases by 4.
E) remains unchanged.
A) increases by 4.
B) increases by 2.
C) decreases by 2.
D) decreases by 4.
E) remains unchanged.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
55
A radioactive isotope of atomic number Z emits a beta-minus particle, and then the daughter nucleus emits a gamma ray. What is the atomic number of the resulting nucleus after both processes?
A) Z - 1
B) Z + 1
C) Z - 2
D) Z - 3
A) Z - 1
B) Z + 1
C) Z - 2
D) Z - 3

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
56
When an unstable nucleus decays by emitting an alpha particle, the atomic number Z of the nucleus
A) increases by 4.
B) increases by 2.
C) decreases by 2.
D) decreases by 4.
E) remains unchanged.
A) increases by 4.
B) increases by 2.
C) decreases by 2.
D) decreases by 4.
E) remains unchanged.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
57
What happens to the half-life of a radioactive substance as we increase its temperature?
A) It does not change.
B) It increases.
C) It decreases at a constant rate.
D) It decreases at an exponential rate.
A) It does not change.
B) It increases.
C) It decreases at a constant rate.
D) It decreases at an exponential rate.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
58
Inside the nucleus, the strongest of the four fundamental forces is
A) the weak nuclear force.
B) the electromagnetic force.
C) the gravitational force.
D) the strong nuclear force.
A) the weak nuclear force.
B) the electromagnetic force.
C) the gravitational force.
D) the strong nuclear force.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
59
Inside the nucleus, the weakest of the four fundamental forces is
A) the weak nuclear force.
B) the electromagnetic force.
C) the gravitational force.
D) the strong nuclear force.
A) the weak nuclear force.
B) the electromagnetic force.
C) the gravitational force.
D) the strong nuclear force.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
60
If the half-life of a material is 45 years, how much of it will be left after 100 years?
A) more than 1/2
B) less than 1/2 but more than 1/4
C) more than 1/4 but less than 1/2
D) less than 1/4 but more than 1/8
E) less than 1/8
A) more than 1/2
B) less than 1/2 but more than 1/4
C) more than 1/4 but less than 1/2
D) less than 1/4 but more than 1/8
E) less than 1/8

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
61
In a head-on collision, an alpha particle (Z = 2) of energy 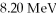 bounces straight back from a nucleus of charge
bounces straight back from a nucleus of charge 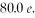 How close were the centers of the objects at closest approach? (1 eV = 1.60 × 10-19 J, e = 1.60 × 10-19 C, 1/4πε0 = 8.99 × 109 N ∙ m2/C2)
How close were the centers of the objects at closest approach? (1 eV = 1.60 × 10-19 J, e = 1.60 × 10-19 C, 1/4πε0 = 8.99 × 109 N ∙ m2/C2)
A) 2.81 × 10-14 m
B) 3.39 × 10-12 m
C) 6.56 × 10-15 m
D) 2.17 × 10-14 m
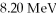 bounces straight back from a nucleus of charge
bounces straight back from a nucleus of charge 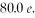 How close were the centers of the objects at closest approach? (1 eV = 1.60 × 10-19 J, e = 1.60 × 10-19 C, 1/4πε0 = 8.99 × 109 N ∙ m2/C2)
How close were the centers of the objects at closest approach? (1 eV = 1.60 × 10-19 J, e = 1.60 × 10-19 C, 1/4πε0 = 8.99 × 109 N ∙ m2/C2)A) 2.81 × 10-14 m
B) 3.39 × 10-12 m
C) 6.56 × 10-15 m
D) 2.17 × 10-14 m

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
62
A beryllium-8 nucleus at rest undergoes double alpha decay as follows: 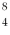 Be →
Be → 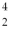 He +
He + 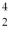 He The known atomic masses are:
He The known atomic masses are: 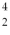 He: 4.002603 u
He: 4.002603 u 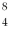 Be: 8.005305 u
Be: 8.005305 u
What is the kinetic energy of each departing alpha particle? (1 u = 931.5 MeV/c2)
A) 46 keV
B) 65 keV
C) 92 keV
D) 130 keV
E) 180 keV
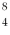 Be →
Be → 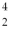 He +
He + 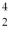 He The known atomic masses are:
He The known atomic masses are: 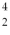 He: 4.002603 u
He: 4.002603 u 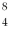 Be: 8.005305 u
Be: 8.005305 uWhat is the kinetic energy of each departing alpha particle? (1 u = 931.5 MeV/c2)
A) 46 keV
B) 65 keV
C) 92 keV
D) 130 keV
E) 180 keV

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
63
What combination of quarks produces a proton and what are the electric charges on these quarks, expressed in terms of e?

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
64
What are the possible charges of a quark (not an antiquark)?
A) -e, 0, e
B) -2/3 e, -1/3 e, +1/3 e, +2/3 e
C) -2/3 e, +1/3 e
D) -1/3 e, +2/3 e
E) -1/3 e, +1/3 e
A) -e, 0, e
B) -2/3 e, -1/3 e, +1/3 e, +2/3 e
C) -2/3 e, +1/3 e
D) -1/3 e, +2/3 e
E) -1/3 e, +1/3 e

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
65
In the first nuclear reaction observed, physicists saw an alpha particle (Z = 2) interact with a nitrogen nucleus in air (Z = 7) to produce a proton. The energy of the alpha particle was 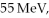 enough to enable the nuclei to touch in spite of the Coulomb repulsion. What distance (in fm) between the centers of the alpha particle and the nitrogen was reached? This determines a limit on the radius of the nitrogen nucleus. (1 eV = 1.60 × 10-19 J, e = 1.60 × 10-19 C, 1/4πε0 = 8.99 × 109 N ∙ m2/C2)
enough to enable the nuclei to touch in spite of the Coulomb repulsion. What distance (in fm) between the centers of the alpha particle and the nitrogen was reached? This determines a limit on the radius of the nitrogen nucleus. (1 eV = 1.60 × 10-19 J, e = 1.60 × 10-19 C, 1/4πε0 = 8.99 × 109 N ∙ m2/C2)
A) 0 fm
B) 0 fm
C) 0 fm
D) 0 fm
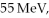 enough to enable the nuclei to touch in spite of the Coulomb repulsion. What distance (in fm) between the centers of the alpha particle and the nitrogen was reached? This determines a limit on the radius of the nitrogen nucleus. (1 eV = 1.60 × 10-19 J, e = 1.60 × 10-19 C, 1/4πε0 = 8.99 × 109 N ∙ m2/C2)
enough to enable the nuclei to touch in spite of the Coulomb repulsion. What distance (in fm) between the centers of the alpha particle and the nitrogen was reached? This determines a limit on the radius of the nitrogen nucleus. (1 eV = 1.60 × 10-19 J, e = 1.60 × 10-19 C, 1/4πε0 = 8.99 × 109 N ∙ m2/C2)A) 0 fm
B) 0 fm
C) 0 fm
D) 0 fm

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
66
The proton is made up of which one of the following quark combinations (up, down, strange, charm, top, bottom)?
A) uud
B) ddu
C) udd
D) ttb
E) bst
A) uud
B) ddu
C) udd
D) ttb
E) bst

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
67
What combination of quarks produces a neutron and what are the electric charges on these quarks, expressed in terms of e?

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
68
A summary of the nuclear reactions that power our sun is 4p → 4He + 2e-, with masses of 938.272 MeV/c2 for a proton, 3727.38 MeV/c2 for the helium nucleus, and 0.511 MeV/c2 for each electron. How much energy is released by each of these reactions?
A) 24.69 MeV
B) 28.3 MeV
C) 2790.13 MeV
D) 279.01 MeV
A) 24.69 MeV
B) 28.3 MeV
C) 2790.13 MeV
D) 279.01 MeV

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
69
The neutron is made up of which one of the following quark combinations (up, down, strange, charm, top, bottom)?
A) uud
B) ddu
C) udd
D) ttb
E) bst
A) uud
B) ddu
C) udd
D) ttb
E) bst

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following particles are leptons? (There may be more than one correct choice.)
A) protons
B) neutrons
C) electrons
D) photons
E) quarks
A) protons
B) neutrons
C) electrons
D) photons
E) quarks

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
71
Elementary particles that experience the weak nuclear force but not the strong nuclear force are called
A) leptons.
B) hadrons.
C) mesons.
D) bosons.
E) baryons.
A) leptons.
B) hadrons.
C) mesons.
D) bosons.
E) baryons.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
72
One of the fusion reactions that occurs in the sun is: 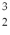 He +
He + 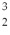 He →
He → 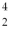 He +
He + 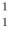 H +
H + 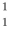 H The following atomic masses are known:
H The following atomic masses are known: 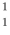 H: 1.007825 u
H: 1.007825 u 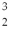 He: 3.016029 u
He: 3.016029 u 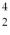 He: 4.002603 u
He: 4.002603 u
What is the reaction energy released in this fusion reaction? (1 u = 931.5 MeV/c2)
A) 11 MeV
B) 13 MeV
C) 15 MeV
D) 17 MeV
E) 19 MeV
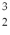 He +
He + 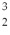 He →
He → 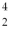 He +
He +  H +
H +  H The following atomic masses are known:
H The following atomic masses are known:  H: 1.007825 u
H: 1.007825 u 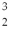 He: 3.016029 u
He: 3.016029 u 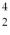 He: 4.002603 u
He: 4.002603 uWhat is the reaction energy released in this fusion reaction? (1 u = 931.5 MeV/c2)
A) 11 MeV
B) 13 MeV
C) 15 MeV
D) 17 MeV
E) 19 MeV

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following particles are not made up of quarks? (There could be more than one correct choice.)
A) alpha particle
B) electron
C) proton
D) positron
E) neutron
A) alpha particle
B) electron
C) proton
D) positron
E) neutron

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following particles (or groups of particles) are made up of quarks?
A) protons, neutrons, and electrons
B) electrons and neutrinos
C) photons
D) protons and neutrons
E) All particles except for photons are made up of quarks.
A) protons, neutrons, and electrons
B) electrons and neutrinos
C) photons
D) protons and neutrons
E) All particles except for photons are made up of quarks.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
75
How many quarks are in a deuteron, 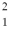 H?
H?
A) 2
B) 3
C) 4
D) 6
E) 9
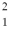 H?
H?A) 2
B) 3
C) 4
D) 6
E) 9

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
76
How many quarks are in a tritium isotope, 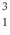 H?
H?
A) 2
B) 3
C) 4
D) 6
E) 9
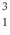 H?
H?A) 2
B) 3
C) 4
D) 6
E) 9

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
77
Leptons can interact by which of the following forces?
A) strong nuclear force, weak nuclear force, electromagnetic force, gravitation
B) strong nuclear force, weak nuclear force, electromagnetic force
C) weak nuclear force, electromagnetic force, gravitation
D) strong nuclear force, weak nuclear force
E) strong nuclear force, electromagnetic force, gravitation
A) strong nuclear force, weak nuclear force, electromagnetic force, gravitation
B) strong nuclear force, weak nuclear force, electromagnetic force
C) weak nuclear force, electromagnetic force, gravitation
D) strong nuclear force, weak nuclear force
E) strong nuclear force, electromagnetic force, gravitation

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
78
The diameter of an atom is 1.4 × 10-10 m and the diameter of its nucleus is 1.0 × 10-14 m. What fraction of the atom's volume is occupied by its nucleus?
A) 3.6 × 10-13
B) 7.1 × 10-5
C) 5.1 × 10-9
D) 2.6 × 10-17
A) 3.6 × 10-13
B) 7.1 × 10-5
C) 5.1 × 10-9
D) 2.6 × 10-17

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
79
How much energy is released when 0.60 μg of 3H have decayed to 3He? The mass of 3He is 3.0160293 u, the mass of 3H is 3.0160492 u, 1 u = 931.5 MeV/c2, 1 eV = 1.60 × 10-19 J, and NA = 6.022 × 1023 molecules/mol.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
80
The radius R of a nucleus of mass number A is given by R = R0 A1/3, where R0 = 1.2 × 10-15 m, calculate the density of a nucleus that has contains 57 protons and 82 neutrons. The mass of a nucleon (proton or neutron) is 1.67 × 10-27 kg.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck


