Deck 13: First Law of Thermodynamics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/119
Play
Full screen (f)
Deck 13: First Law of Thermodynamics
1
An ideal gas is compressed isothermally to one-third of its initial volume.The resulting pressure will be
A)three times as large as the initial value.
B)less than three times as large as the initial value.
C)more than three times as large as the initial value.
D)equal to the initial value.
E)impossible to predict on the basis of this data.
A)three times as large as the initial value.
B)less than three times as large as the initial value.
C)more than three times as large as the initial value.
D)equal to the initial value.
E)impossible to predict on the basis of this data.
A
2
A solid cylindrical bar conducts thermal energy at a rate of 25 W from a hot to a cold reservoir under steady state conditions.If both the length and the diameter of this bar are doubled,the rate at which it will conduct thermal energy between these reservoirs will be
A)200 W
B)100 W
C)50 W
D)25 W
E)12.5 W
A)200 W
B)100 W
C)50 W
D)25 W
E)12.5 W
C
3
The figure shows a graph of the temperature of a pure substance as a function of time as energy through heating is added to it at a constant rate in a closed container.If LF is the latent heat of fusion of this substance and LV is its latent heat of vaporization,what is the value of the ratio LV/LF? 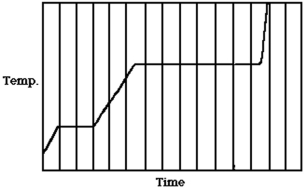
A)5.0
B)4.5
C)7.2
D)3.5
E)1.5

A)5.0
B)4.5
C)7.2
D)3.5
E)1.5
D
4
Object 1 has three times the specific heat capacity and four times the mass of Object 2.The two objects are heated from the same initial temperature,T0,to the same final temperature Tf.If during this process,if Object 1 absorbs energy through heating Q,the amount of energy absorbed through heating by Object 2 will be
A)12Q.
B)6Q.
C) Q.
Q.
D) Q.
Q.
E)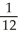 Q.
Q.
A)12Q.
B)6Q.
C)
 Q.
Q.D)
 Q.
Q.E)
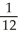 Q.
Q.
Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
5
The process shown on the pV diagram in the figure is an 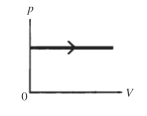
A)adiabatic expansion.
B)isothermal expansion.
C)isometric expansion.
D)isobaric expansion.

A)adiabatic expansion.
B)isothermal expansion.
C)isometric expansion.
D)isobaric expansion.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
6
Object 1 has three times the specific heat capacity and four times the mass of Object 2.The same amount of energy is transferred to each object though the process of heating.If the temperature of Object 1 changes by an amount ΔT,the change in temperature of Object 2 will be
A)ΔT.
B) ΔT.
ΔT.
C) ΔT.
ΔT.
D)6ΔT.
E)12ΔT.
A)ΔT.
B)
 ΔT.
ΔT.C)
 ΔT.
ΔT.D)6ΔT.
E)12ΔT.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
7
If,with steady state thermal energy flow established,you double the thickness of a wall built from solid uniform material,the rate of thermal energy transfer our of the room for a given temperature difference across the thickness will
A)become one-half its original value.
B)also double.
C)become one-fourth its original value.
D)become 1/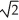 of its original value.
of its original value.
E)become four times its original value.
A)become one-half its original value.
B)also double.
C)become one-fourth its original value.
D)become 1/
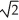 of its original value.
of its original value.E)become four times its original value.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
8
An architect is interested in estimating the rate of energy loss through heating,ΔQ/Δt,through a sheet of insulating material as a function of the thickness of the sheet.Assuming fixed temperatures on the two faces of the sheet and steady state thermal energy flow,which one of the graphs shown in the figure best represents the rate of thermal energy transfer as a function of the thickness of the insulating sheet? 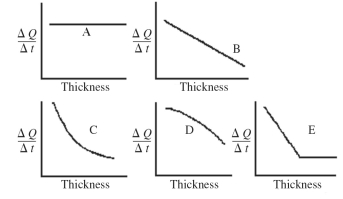
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
9
A thermally isolated system is made up of a hot piece of aluminum and a cold piece of copper,with the aluminum and the copper in thermal contact.The specific heat capacity of aluminum is more than double that of copper.Which object will have the greater magnitude gain or loss of internal energy during the time the system takes to reach thermal equilibrium?
A)the aluminum
B)the copper
C)Neither one; both of them will have the same size gain or loss of internal energy.
D)It is impossible to tell without knowing the masses.
E)It is impossible to tell without knowing the volumes.
A)the aluminum
B)the copper
C)Neither one; both of them will have the same size gain or loss of internal energy.
D)It is impossible to tell without knowing the masses.
E)It is impossible to tell without knowing the volumes.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
10
The process in which thermal energy flows by the mass movement of molecules from one place to another is known as
A)conduction.
B)convection.
C)radiation.
A)conduction.
B)convection.
C)radiation.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
11
In a given reversible process,the temperature of an ideal gas is kept constant as the gas is compressed to a smaller volume.Which one of the following statements about the gas is correct?
A)The gas must absorb thermal energy through the process of heating from its surroundings.
B)The gas must release thermal energy by heating to its surroundings.
C)The pressure of the gas also stays constant.
D)The process is adiabatic.
E)It is impossible to predict on the basis of this data.
A)The gas must absorb thermal energy through the process of heating from its surroundings.
B)The gas must release thermal energy by heating to its surroundings.
C)The pressure of the gas also stays constant.
D)The process is adiabatic.
E)It is impossible to predict on the basis of this data.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
12
The process shown on the pV diagram in the figure is 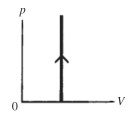
A)adiabatic.
B)isothermal.
C)isochoric.
D)isobaric.

A)adiabatic.
B)isothermal.
C)isochoric.
D)isobaric.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
13
An ideal gas is compressed isobarically to one-third of its initial volume.The resulting pressure will be
A)three times as large as the initial value.
B)equal to the initial value.
C)more than three times as large as the initial value.
D)nine times the initial value.
E)impossible to predict on the basis of this data.
A)three times as large as the initial value.
B)equal to the initial value.
C)more than three times as large as the initial value.
D)nine times the initial value.
E)impossible to predict on the basis of this data.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
14
A thermally isolated system is made up of a hot piece of aluminum and a cold piece of copper,with the aluminum and the copper in thermal contact.The specific heat capacity of aluminum is more than double that of copper.Which object will have the greater temperature change during the time the system takes to reach thermal equilibrium?
A)the copper
B)the aluminum
C)Neither one; both of them will have the same size temperature change.
D)It is impossible to tell without knowing the masses.
E)It is impossible to tell without knowing the volumes.
A)the copper
B)the aluminum
C)Neither one; both of them will have the same size temperature change.
D)It is impossible to tell without knowing the masses.
E)It is impossible to tell without knowing the volumes.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
15
If you wanted to know how much the temperature of a particular piece of material would rise when a known amount of energy through the process of heating was added to it,which of the following quantities would be most helpful to know?
A)initial temperature
B)specific heat
C)density
D)coefficient of linear expansion
E)thermal conductivity
A)initial temperature
B)specific heat
C)density
D)coefficient of linear expansion
E)thermal conductivity

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
16
Which one of the following quantities is the smallest unit of thermal energy?
A)calorie
B)kilocalorie
C)Btu
D)joule
A)calorie
B)kilocalorie
C)Btu
D)joule

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
17
Two processes are shown on the pV diagram in the figure.One of them is an adiabat and the other one is an isotherm.Which process is the isotherm? 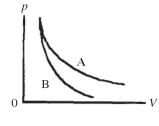
A)process A
B)process B
C)The processes shown are neither isotherms nor adiabats.
D)It is not possible to tell without knowing if the gas is monatomic or diatomic.

A)process A
B)process B
C)The processes shown are neither isotherms nor adiabats.
D)It is not possible to tell without knowing if the gas is monatomic or diatomic.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
18
The process shown on the TV graph in the figure is an 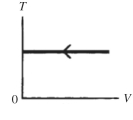
A)adiabatic compression.
B)isothermal compression.
C)isochoric compression.
D)isobaric compression.

A)adiabatic compression.
B)isothermal compression.
C)isochoric compression.
D)isobaric compression.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
19
By what primary thermal energy transfer mechanism does the Sun warm Earth?
A)convection
B)conduction
C)radiation
D)All of the above processes are equally important in combination.
A)convection
B)conduction
C)radiation
D)All of the above processes are equally important in combination.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
20
On a cold day,a piece of metal feels much colder to the touch than a piece of wood.This is due to the difference in which one of the following physical properties of these materials?
A)density
B)specific heat
C)emissivity
D)thermal conductivity
E)mass
A)density
B)specific heat
C)emissivity
D)thermal conductivity
E)mass

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
21
It is necessary to determine the specific heat of an unknown object.The mass of the object is 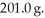 It is determined experimentally that it takes
It is determined experimentally that it takes 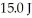 to raise the temperature
to raise the temperature 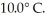 What is the specific heat of the object?
What is the specific heat of the object?
A)7.46 J/kg ∙ K
B)1500 J/kg ∙ K
C)0.00130 J/kg ∙ K
D)3,020,000 J/kg ∙ K
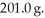 It is determined experimentally that it takes
It is determined experimentally that it takes 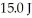 to raise the temperature
to raise the temperature 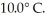 What is the specific heat of the object?
What is the specific heat of the object?A)7.46 J/kg ∙ K
B)1500 J/kg ∙ K
C)0.00130 J/kg ∙ K
D)3,020,000 J/kg ∙ K

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
22
A cyclic process is carried out on an ideal gas such that it returns to its initial state at the end of a cycle,as shown in the pV diagram in the figure.If the process is carried out in a clockwise sense around the enclosed area,as shown on the figure,then the change of internal energy over the full cycle 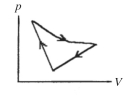
A)is positive.
B)is negative.
C)is zero.
D)cannot be determined from the information given.

A)is positive.
B)is negative.
C)is zero.
D)cannot be determined from the information given.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
23
If 150 kcal of thermal energy raises the temperature of 2.0 kg of a material by 400 F°,what is the specific heat capacity of the material?
A)1.35 kcal/kg ∙ C°
B)0.75 kcal/kg ∙ C°
C)0.34 kcal/kg ∙ C°
D)0.19 kcal/kg ∙ C°
A)1.35 kcal/kg ∙ C°
B)0.75 kcal/kg ∙ C°
C)0.34 kcal/kg ∙ C°
D)0.19 kcal/kg ∙ C°

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
24
A gas is taken through the cycle shown in the pV diagram in the figure.During one cycle,how much work is done by the gas? 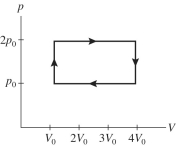
A)p0V0
B)2 p0V0
C)3 p0V0
D)4 p0V0

A)p0V0
B)2 p0V0
C)3 p0V0
D)4 p0V0

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
25
An aluminum electric tea kettle with a mass of 500 g is heated with a 500-W heating coil.How long will it take to change the temperature of 1.0 kg of water from 18°C to 98°C in the tea kettle? The specific heat of aluminum is 900 J/kg ∙ K and that of water is 4186 J/kg ∙ K.
A)5.0 minutes
B)7.0 minutes
C)12 minutes
D)15 minutes
E)18 minutes
A)5.0 minutes
B)7.0 minutes
C)12 minutes
D)15 minutes
E)18 minutes

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
26
A 6.5-g iron meteor hits Earth at a speed of 295 m/s.If its kinetic energy is entirely converted to the thermal energy of the meteor,by how much will its temperature rise? The specific heat of iron is 113 cal/kg ∙ C°,and 1 cal = 4.186 J.
A)92.0 C°
B)57,100 C°
C)0.147 C°
D)384 C°
A)92.0 C°
B)57,100 C°
C)0.147 C°
D)384 C°

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
27
At room temperature,a typical person transfers energy to the surroundings at the rate of 62 W.If this energy transfer has to be made up by an equivalent food intake,how many kilocalories (food calories)does this person need to consume every day just to make up this energy? (1 cal = 4.186 J)
A)1000 kcal
B)1100 kcal
C)1300 kcal
D)1500 kcal
E)1600 kcal
A)1000 kcal
B)1100 kcal
C)1300 kcal
D)1500 kcal
E)1600 kcal

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
28
The water flowing over Niagara Falls drops a distance of 50 m.If all the gravitational potential energy of the system water-Earth is converted to thermal energy,by what temperature does the water rise? The specific heat of water is 4186 J/kg ∙ K.
A)0.10 C°
B)0.12 C°
C)0.37 C°
D)0.42 C°
A)0.10 C°
B)0.12 C°
C)0.37 C°
D)0.42 C°

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
29
A 920-g empty iron kettle is put on a stove.How much thermal energy in joules must be transferred to it to raise its temperature form 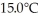 to
to 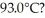 The specific heat for iron is 113 cal/kg ∙ C°,and 1 cal = 4.186 J.
The specific heat for iron is 113 cal/kg ∙ C°,and 1 cal = 4.186 J.
A)33,900 J
B)40,500 J
C)8110 J
D)40,100 J
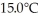 to
to 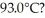 The specific heat for iron is 113 cal/kg ∙ C°,and 1 cal = 4.186 J.
The specific heat for iron is 113 cal/kg ∙ C°,and 1 cal = 4.186 J.A)33,900 J
B)40,500 J
C)8110 J
D)40,100 J

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
30
A person consumes a snack containing 14 food calories (14 kcal).What is the power this food produces if it is to be "burned off" due to exercise in 6 hours? (1 cal = 4.186 J)
A)2.7 W
B)9763 W
C)0.6 W
D)0.0027 W
A)2.7 W
B)9763 W
C)0.6 W
D)0.0027 W

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
31
On his honeymoon,James Joule attempted to explore the relationships between various forms of energy by measuring the rise of temperature of water which had fallen down a waterfall on Mount Blanc.What maximum temperature rise would one expect for a waterfall with a vertical drop of 20 m? The specific heat of water is 4186 J/kg ∙ K.
A)0.047 C°
B)0.053 C°
C)0.064 C°
D)0.071 C°
A)0.047 C°
B)0.053 C°
C)0.064 C°
D)0.071 C°

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
32
A carpenter is driving a 15.0-g steel nail into a board.His 1.00-kg hammer is moving at 8.50 m/s when it strikes the nail.Half of the kinetic energy of the hammer is converted into internal energyin the nail and does not flow out of the nail.What is the increase in temperature of the nail after the three blows that the carpenter needs to drive the nail in completely? The specific heat of steel is 448 J/kg ∙ K.
A)8.1 K
B)3.6 K
C)1.8 K
D)2.7 K
E)7.7 K
A)8.1 K
B)3.6 K
C)1.8 K
D)2.7 K
E)7.7 K

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
33
A machine part consists of 0.10 kg of iron (of specific heat 470 J/kg ∙ K )and 0.16 kg of copper (of specific heat 390 J/kg ∙ K).How much thermal energy must be added to the gear to raise its temperature from 18°C to 53°C?
A)910 J
B)3800 J
C)4000 J
D)4400 J
A)910 J
B)3800 J
C)4000 J
D)4400 J

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
34
A 4.0-kg aluminum block is originally at 10°C.If 160 kJ of thermal energy is added to the block,what is its final temperature? The specific heat capacity of aluminum is 910 J/kg ∙ K.
A)24°C
B)34°C
C)44°C
D)54°C
A)24°C
B)34°C
C)44°C
D)54°C

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
35
A 5.00-g lead BB moving at 44.0 m/s penetrates a wood block and comes to rest inside the block.If half of its kinetic energy is absorbed by the BB,what is the change in the temperature of the BB? The specific heat of lead is 128 J/kg ∙ K.
A)0.940 K
B)1.10 K
C)1.26 K
D)2.78 K
E)3.78 K
A)0.940 K
B)1.10 K
C)1.26 K
D)2.78 K
E)3.78 K

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
36
A certain gas is compressed adiabatically.The amount of work done on the gas is 800 J.What is the change in the internal (thermal)energy of the gas?
A)800 J
B)-800 J
C)400 J
D)0 J
E)More information is needed to answer this question.
A)800 J
B)-800 J
C)400 J
D)0 J
E)More information is needed to answer this question.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
37
A person running in place on an exercise machine for 10 min uses up 17 kcal (food calories).Another person exercises by repeatedly lifting two 2.5-kg weights a distance of 50 cm.How many repetitions of this exercise are equivalent to 10 minutes of running in place? Assume that the person uses negligible energy in letting down the weights after each lift.(1 cal = 4.186 J)
A)730
B)1450
C)1500
D)2200
E)2900
A)730
B)1450
C)1500
D)2200
E)2900

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
38
A container of 114.0 g of water is heated using 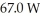 of power,with perfect efficiency.How long will it take to raise the temperature of the water from
of power,with perfect efficiency.How long will it take to raise the temperature of the water from 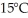 to
to 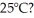 The specific heat of the container is negligible,and the specific heat of water is 4.186 × 103 J/kg ∙ C.
The specific heat of the container is negligible,and the specific heat of water is 4.186 × 103 J/kg ∙ C.
A)71 s
B)4.1 s
C)17 s
D)320,000 s
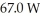 of power,with perfect efficiency.How long will it take to raise the temperature of the water from
of power,with perfect efficiency.How long will it take to raise the temperature of the water from 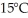 to
to 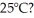 The specific heat of the container is negligible,and the specific heat of water is 4.186 × 103 J/kg ∙ C.
The specific heat of the container is negligible,and the specific heat of water is 4.186 × 103 J/kg ∙ C.A)71 s
B)4.1 s
C)17 s
D)320,000 s

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
39
A glass beaker of unknown mass contains 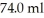 of water.The system absorbs
of water.The system absorbs 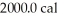 of thermal energy and the temperature rises
of thermal energy and the temperature rises 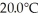 as a result.What is the mass of the beaker? The specific heat of glass is 0.18 cal/g ∙ °C,and that of water is 1.0 cal/g ∙ C°.
as a result.What is the mass of the beaker? The specific heat of glass is 0.18 cal/g ∙ °C,and that of water is 1.0 cal/g ∙ C°.
A)140 g
B)560 g
C)540 g
D)270,000 g
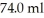 of water.The system absorbs
of water.The system absorbs 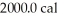 of thermal energy and the temperature rises
of thermal energy and the temperature rises 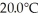 as a result.What is the mass of the beaker? The specific heat of glass is 0.18 cal/g ∙ °C,and that of water is 1.0 cal/g ∙ C°.
as a result.What is the mass of the beaker? The specific heat of glass is 0.18 cal/g ∙ °C,and that of water is 1.0 cal/g ∙ C°.A)140 g
B)560 g
C)540 g
D)270,000 g

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
40
How much thermal energy is required to raise the temperature of a 225-g lead ball from 15.0°C to 25.0°C? The specific heat of lead is 128 J/kg ∙ K.
A)725 J
B)576 J
C)145 J
D)217 J
E)288 J
A)725 J
B)576 J
C)145 J
D)217 J
E)288 J

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
41
In grinding a steel knife,the metal can get as hot as 400°C.If the blade has a mass of 80 g,what is the minimum amount of water needed at 20°C if the water is to remain liquid and not rise above 100°C when the hot blade is quenched in it? The specific heat of the steel is 0.11 cal/g ∙ C° and the specific heat of water is 1.0 cal/g ∙ C°.
A)22 g
B)33 g
C)44 g
D)55 g
A)22 g
B)33 g
C)44 g
D)55 g

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
42
A beaker of negligible heat capacity contains 456 g of ice at -25.0°C.A lab technician begins to supply thermal energy through the process of heating to the container at the rate of 1000 J/min.How long after starting will the ice begin to melt,assuming all of the ice has the same temperature? The specific heat of ice is 2090 J/kg ∙ K and the latent heat of fusion of water is 33.5 × 104 J/kg.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
43
A 771.0-kg copper bar is put into a smelter for melting.The initial temperature of the copper is 300.0 K.How much thermal energy must the smelter produce to completely melt the copper bar? The specific heat for copper is 386 J/kg∙K,the heat of fusion for copper is 205,000 J/kg,and its melting point is 1357 K.
A)4.73 × 105 kJ
B)3.15 × 1011 kJ
C)3.15 × 108 kJ
D)5.62 × 105 kJ
A)4.73 × 105 kJ
B)3.15 × 1011 kJ
C)3.15 × 108 kJ
D)5.62 × 105 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
44
If you add 700 kJ of thermal energy to 700 g of water originally at 70.0°C,how much water is left in the container? The latent heat of vaporization of water is 22.6 × 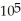 J/kg,and its specific heat capacity is 4186 J/kg ∙ K.
J/kg,and its specific heat capacity is 4186 J/kg ∙ K.
A)429 g
B)258 g
C)340 g
D)600 g
E)none
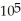 J/kg,and its specific heat capacity is 4186 J/kg ∙ K.
J/kg,and its specific heat capacity is 4186 J/kg ∙ K.A)429 g
B)258 g
C)340 g
D)600 g
E)none

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
45
A 45.0-kg sample of ice is at 0.00° C.How much thermal energy is needed to melt it? For water LF = 334,000 J/kg and LV = 2.256 × 106 J/kg.
A)1.50 × 104 kJ
B)4.10 × 106 kJ
C)0.00 kJ
D)1.02 × 105 kJ
A)1.50 × 104 kJ
B)4.10 × 106 kJ
C)0.00 kJ
D)1.02 × 105 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
46
A .20-kg ice cube at 0.0°C has sufficient thermal energy added to it through the process of heating to cause total melting,and the resulting water is heated to 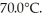 How much energy is added? For water LF = 334,000 J/kg,LV = 2.256 × 106 J/kg,the c = 4.186 x 103 J/kg ∙ C.
How much energy is added? For water LF = 334,000 J/kg,LV = 2.256 × 106 J/kg,the c = 4.186 x 103 J/kg ∙ C.
A)130 kJ
B)14,000 kJ
C)81 kJ
D)59 kJ
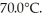 How much energy is added? For water LF = 334,000 J/kg,LV = 2.256 × 106 J/kg,the c = 4.186 x 103 J/kg ∙ C.
How much energy is added? For water LF = 334,000 J/kg,LV = 2.256 × 106 J/kg,the c = 4.186 x 103 J/kg ∙ C.A)130 kJ
B)14,000 kJ
C)81 kJ
D)59 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
47
If you add 1.33 MJ of thermal energy to 500 g of water at 50°C in a sealed container,what is the final temperature of the steam? The latent heat of vaporization of water is 22.6 × 105 J/kg,the specific heat of steam is 2010 J/kg ∙ K,and the specific heat of water is 4186 J/kg ∙ K.
A)100°C
B)112°C
C)123°C
D)147°C
E)195°C
A)100°C
B)112°C
C)123°C
D)147°C
E)195°C

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
48
A metal has a latent heat of fusion of 2.32 × 104 J/kg,a specific heat of 128 J/kg ∙ K,and a melting point of 228°C.A 30-g pellet of this metal at 16°C hits a solid wall and comes to a complete stop.What would the speed of the pellet have to be in order for it to melt completely when it hits the wall,assuming that all of its kinetic energy is transformed into thermal energy within the pellet?
A)207 m/s
B)215 m/s
C)232 m/s
D)273 m/s
E)317 m/s
A)207 m/s
B)215 m/s
C)232 m/s
D)273 m/s
E)317 m/s

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
49
In a flask,114.0 g of water is heated using 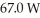 of power,with perfect efficiency.How long will it take to raise the temperature of the water from
of power,with perfect efficiency.How long will it take to raise the temperature of the water from 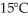 to
to 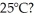 The specific heat of water is 4186 J/kg ∙ K.
The specific heat of water is 4186 J/kg ∙ K.
A)71 s
B)4.1 s
C)17 s
D)320,000 s
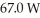 of power,with perfect efficiency.How long will it take to raise the temperature of the water from
of power,with perfect efficiency.How long will it take to raise the temperature of the water from 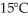 to
to 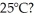 The specific heat of water is 4186 J/kg ∙ K.
The specific heat of water is 4186 J/kg ∙ K.A)71 s
B)4.1 s
C)17 s
D)320,000 s

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
50
Solar houses use a variety of energy storage devices to retain the thermal energy absorbed during the day so that it can be released during the night.Suppose that you were to use a device of this kind to produce steam at 100°C during the day,and then allow the steam to cool to 0°C and freeze during the night.How many kilograms of water would be needed to store 20.0 kWh of energy in this way? The latent heat of vaporization of water is 22.6 × 105 J/kg,the latent heat of fusion of water is 33.5 × 104 J/kg,and the specific heat of water is 4186 J/kg ∙ K.
A)12.4 kg
B)23.9 kg
C)35.7 kg
D)42.6 kg
E)54.2 kg
A)12.4 kg
B)23.9 kg
C)35.7 kg
D)42.6 kg
E)54.2 kg

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
51
A 200-L electric water heater uses 2.0 kW.Assuming no thermal energy loss,how many hours would it take to change the temperature of water in this tank from 23°C to 75°C? The specific heat of water is 4186 J/kg ∙ K and its density is 1000 kg/m3.
A)5.0. hours
B)6.0 hours
C)7.0 hours
D)8.0 hours
A)5.0. hours
B)6.0 hours
C)7.0 hours
D)8.0 hours

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
52
The melting point of aluminum is 660°C,its latent heat of fusion is 4.00 × 105 J/kg,and its specific heat is 900 J/kg ∙ K.If 300 kJ of thermal energy are added to 442 g of aluminum at 100°C,what is the final state of the system? That is,how much is liquid,how much is solid,and what is its temperature?

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
53
How much thermal energy must be removed from 456 g of water at 25.0°C to change it into ice at -10.0°C? The specific heat of ice is 2090 J/kg ∙ K,the latent heat of fusion of water is 33.5 × 104 J/kg,and the specific heat of water is 4186 J/kg ∙ K.
A)105 kJ
B)153 kJ
C)57.3 kJ
D)47.7 kJ
E)210 kJ
A)105 kJ
B)153 kJ
C)57.3 kJ
D)47.7 kJ
E)210 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
54
A beaker of negligible heat capacity contains 456 g of ice at -25.0°C.A lab technician begins to supply thermal energy through the process of heating to the container at the rate of 1000 J/min.How long after starting will it take before the temperature starts to rise above 0°C? The specific heat of ice is 2090 J/kg ∙ K and the latent heat of fusion of water is 33.5 × 104 J/kg.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
55
Thermal energy through the process of heating is added to a 3.0 kg piece of ice at a rate of 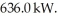 How long will it take for the ice at 0.0° C to melt? For water LF = 334,000 J/kg and LV = 2.246 × 106 J/kg.
How long will it take for the ice at 0.0° C to melt? For water LF = 334,000 J/kg and LV = 2.246 × 106 J/kg.
A)1.6 s
B)640,000 s
C)0.0 s
D)1000 s
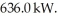 How long will it take for the ice at 0.0° C to melt? For water LF = 334,000 J/kg and LV = 2.246 × 106 J/kg.
How long will it take for the ice at 0.0° C to melt? For water LF = 334,000 J/kg and LV = 2.246 × 106 J/kg.A)1.6 s
B)640,000 s
C)0.0 s
D)1000 s

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
56
When a sample of water at 0.0°C is cooled to -36.0°C and freezes in the process,935,000 kJ of thermal energy is liberated.What is the mass of this sample of water? For water LF = 334,000 J/kg,LV = 2.256 × 106 J/kg,and the specific heat of ice is 2050 J/kg ∙ C°.
A)2290 kg
B)1145 kg
C)2800 kg
D)12,700 kg
A)2290 kg
B)1145 kg
C)2800 kg
D)12,700 kg

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
57
How much energy must be added to a 8.0-kg block of ice at -8°C to change it to water at 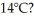 The specific heat of ice is 2050 J/kg ∙ C°,the specific heat of water is 4186 J/kg ∙ C°,the latent heat of fusion of ice is 334,000 J/kg,and 1 cal = 4.186 J.
The specific heat of ice is 2050 J/kg ∙ C°,the specific heat of water is 4186 J/kg ∙ C°,the latent heat of fusion of ice is 334,000 J/kg,and 1 cal = 4.186 J.
A)780 kcal
B)140 kcal
C)180 kcal
D)810 kcal
E)730 kcal
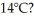 The specific heat of ice is 2050 J/kg ∙ C°,the specific heat of water is 4186 J/kg ∙ C°,the latent heat of fusion of ice is 334,000 J/kg,and 1 cal = 4.186 J.
The specific heat of ice is 2050 J/kg ∙ C°,the specific heat of water is 4186 J/kg ∙ C°,the latent heat of fusion of ice is 334,000 J/kg,and 1 cal = 4.186 J.A)780 kcal
B)140 kcal
C)180 kcal
D)810 kcal
E)730 kcal

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
58
A 2294-kg sample of water at 0° C is cooled to 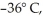 and freezes in the process.How much thermal energy is released? For water LF = 334,000 J/kg and LV = 2.256 × 106 J/kg.The specific heat of ice is 2050 J/kg ∙ K.
and freezes in the process.How much thermal energy is released? For water LF = 334,000 J/kg and LV = 2.256 × 106 J/kg.The specific heat of ice is 2050 J/kg ∙ K.
A)935,000 kJ
B)597,000 kJ
C)1,110,000 kJ
D)334,000 kJ
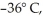 and freezes in the process.How much thermal energy is released? For water LF = 334,000 J/kg and LV = 2.256 × 106 J/kg.The specific heat of ice is 2050 J/kg ∙ K.
and freezes in the process.How much thermal energy is released? For water LF = 334,000 J/kg and LV = 2.256 × 106 J/kg.The specific heat of ice is 2050 J/kg ∙ K.A)935,000 kJ
B)597,000 kJ
C)1,110,000 kJ
D)334,000 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
59
The melting point of aluminum is 660°C,its latent heat of fusion is 4.00 × 105 J/kg,and its specific heat is 900J/kg ∙ K.How much thermal energy must be added to 500 g of aluminum originally at 27°C to completely melt it?
A)485 kJ
B)395 kJ
C)273 kJ
D)147 kJ
E)14 kJ
A)485 kJ
B)395 kJ
C)273 kJ
D)147 kJ
E)14 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
60
A runner generates 1260 W of thermal energy.If this energy has to be removed only by evaporation,how much water does this runner lose in 15 minutes of running? The latent heat of vaporization of water is 22.6 × 105 J/kg.
A)50 g
B)500 g
C)35 g
D)350 g
E)40 g
A)50 g
B)500 g
C)35 g
D)350 g
E)40 g

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
61
A camper is about to drink his morning coffee.He pours 400 grams of coffee,initially at 75°C into a 250-g aluminum cup,initially at 16°C.What is the equilibrium temperature of the coffee-cup system,assuming no thermal energy exchange with the surroundings? The specific heat of aluminum is 900 J/kg ∙ K,and the specific heat of coffee is essentially the same as that of water,which is 4186 J/kg ∙ K.
A)45°C
B)62°C
C)65°C
D)68°C
E)71°C
A)45°C
B)62°C
C)65°C
D)68°C
E)71°C

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
62
A 40.0-g block of ice at -15.00°C is dropped into a calorimeter (of negligible heat capacity)containing water at 15.00°C.When equilibrium is reached,the final temperature is 8.00°C.How much water did the calorimeter contain initially? The specific heat of ice is 2090 J/kg ∙ K,that of water is 4186 J/kg ∙ K,and the latent heat of fusion of water is 33.5 × 104 J/kg.
A)302 g
B)345 g
C)405 g
D)546 g
E)634 g
A)302 g
B)345 g
C)405 g
D)546 g
E)634 g

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
63
A lab assistant drops a 400.0-g piece of metal at 100.0°C into a 100.0-g aluminum cup containing 500.0 g of water at 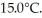 In a few minutes,she measures the final temperature of the system to be 40.0°C.What is the specific heat of the 400.0-g piece of metal,assuming that no significant thermal energy is exchanged with the surroundings? The specific heat of this aluminum is 900.0 J/kg ∙ K and that of water is 4186 J/kg ∙ K.
In a few minutes,she measures the final temperature of the system to be 40.0°C.What is the specific heat of the 400.0-g piece of metal,assuming that no significant thermal energy is exchanged with the surroundings? The specific heat of this aluminum is 900.0 J/kg ∙ K and that of water is 4186 J/kg ∙ K.
A)1900 J/kg ∙ K
B)2270 J/kg ∙ K
C)3300 J/kg ∙ K
D)3800 J/kg ∙ K
E)4280 J/kg ∙ K
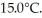 In a few minutes,she measures the final temperature of the system to be 40.0°C.What is the specific heat of the 400.0-g piece of metal,assuming that no significant thermal energy is exchanged with the surroundings? The specific heat of this aluminum is 900.0 J/kg ∙ K and that of water is 4186 J/kg ∙ K.
In a few minutes,she measures the final temperature of the system to be 40.0°C.What is the specific heat of the 400.0-g piece of metal,assuming that no significant thermal energy is exchanged with the surroundings? The specific heat of this aluminum is 900.0 J/kg ∙ K and that of water is 4186 J/kg ∙ K.A)1900 J/kg ∙ K
B)2270 J/kg ∙ K
C)3300 J/kg ∙ K
D)3800 J/kg ∙ K
E)4280 J/kg ∙ K

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
64
A lab assistant pours 330 g of water at 45°C into an 855-g aluminum container that is at an initial temperature of 10°C.The specific heat of aluminum is 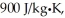 and that of water is 4186 J/kg ∙ K.What is the final temperature of the system,assuming no thermal energy is exchanged with the surroundings?
and that of water is 4186 J/kg ∙ K.What is the final temperature of the system,assuming no thermal energy is exchanged with the surroundings?
A)28°C
B)32°C
C)31°C
D)33°C
E)35°C
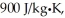 and that of water is 4186 J/kg ∙ K.What is the final temperature of the system,assuming no thermal energy is exchanged with the surroundings?
and that of water is 4186 J/kg ∙ K.What is the final temperature of the system,assuming no thermal energy is exchanged with the surroundings?A)28°C
B)32°C
C)31°C
D)33°C
E)35°C

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
65
A 0.600-kg piece of metal X is heated to 100°C and placed in an aluminum can of mass 0.200-kg which contains 0.500 kg of water initially at 17.3°C.The final equilibrium temperature of the mixture is 20.2°C,what is the specific heat of metal X? The specific heats of water and aluminum are 4186 J/kg ∙ K (water)and 910 J/kg ∙ K (aluminum).
A)140 J/kg ∙ K
B)270 J/kg ∙ K
C)450 J/kg ∙ K
D)900 J/kg ∙ K
A)140 J/kg ∙ K
B)270 J/kg ∙ K
C)450 J/kg ∙ K
D)900 J/kg ∙ K

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
66
A person is walking outdoors on a cold day when the temperature is -20°C.He is breathing at the rate of 16 breaths per minute,and each time he breathes in he inhales 0.0050 m3 of air.At what rate does he lose thermal energy by breathing if the air in his lungs is heated to body temperature (37°C)before it is exhaled? The specific heat of air is 1020 J/kg ∙ K and the density of air is 1.29 kg/m3.
A)60 W
B)90 W
C)100 W
D)150 W
E)300 W
A)60 W
B)90 W
C)100 W
D)150 W
E)300 W

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
67
A jogger is running outdoors on a cold day when the temperature is -20.0°C.She is breathing at the rate of 25 breaths per minute,and each time she breathes in she inhales 0.00450 m3 of air.How much thermal energy does she lose from breathing during 20.0 minutes of jogging if the air in her lungs is heated to her body temperature of 37.0°C before it is exhaled? The specific heat of air is 1020 J/kg ∙ K and the density of air under typical conditions is 1.29 kg/m3.
A)169 kJ
B)278 kJ
C)354 kJ
D)431 kJ
E)543 kJ
A)169 kJ
B)278 kJ
C)354 kJ
D)431 kJ
E)543 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
68
A substance has a melting point of 20°C and a heat of fusion of 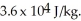 The boiling point is
The boiling point is 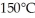 and the heat of vaporization is
and the heat of vaporization is 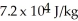 at a pressure of one atmosphere.The specific heats for the solid,liquid,and gaseous phases are 600 J/kg ∙ K (solid),1000 J/kg ∙ K (liquid),and 400 J/kg ∙ K (gaseous).How much thermal energy is emitted by
at a pressure of one atmosphere.The specific heats for the solid,liquid,and gaseous phases are 600 J/kg ∙ K (solid),1000 J/kg ∙ K (liquid),and 400 J/kg ∙ K (gaseous).How much thermal energy is emitted by 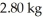 of this substance when it is cooled from 170°C to 86°C at a pressure of one atmosphere?
of this substance when it is cooled from 170°C to 86°C at a pressure of one atmosphere?
A)400 kJ
B)200 kJ
C)300 kJ
D)440 kJ
E)640 kJ
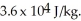 The boiling point is
The boiling point is 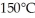 and the heat of vaporization is
and the heat of vaporization is 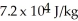 at a pressure of one atmosphere.The specific heats for the solid,liquid,and gaseous phases are 600 J/kg ∙ K (solid),1000 J/kg ∙ K (liquid),and 400 J/kg ∙ K (gaseous).How much thermal energy is emitted by
at a pressure of one atmosphere.The specific heats for the solid,liquid,and gaseous phases are 600 J/kg ∙ K (solid),1000 J/kg ∙ K (liquid),and 400 J/kg ∙ K (gaseous).How much thermal energy is emitted by 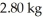 of this substance when it is cooled from 170°C to 86°C at a pressure of one atmosphere?
of this substance when it is cooled from 170°C to 86°C at a pressure of one atmosphere?A)400 kJ
B)200 kJ
C)300 kJ
D)440 kJ
E)640 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
69
A 35-g block of ice at -14°C is dropped into a calorimeter (of negligible heat capacity)containing 400 g of water at 0°C.When the system reaches equilibrium,how much ice is left in the calorimeter? The specific heat of ice is 2090 J/kg ∙ K,that of water is 4186 J/kg ∙ K,and the latent heat of fusion of water is 33.5 × 104 J/kg.
A)32 g
B)33 g
C)35 g
D)38 g
E)41 g
A)32 g
B)33 g
C)35 g
D)38 g
E)41 g

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
70
A person tries to heat up her bath water by adding 5.0 L of water at 80°C to 60 L of water at 30°C.What is the final temperature of the bath water?
A)34°C
B)36°C
C)38°C
D)40°C
A)34°C
B)36°C
C)38°C
D)40°C

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
71
A 44.0-g block of ice at -15.0°C is dropped into a calorimeter (of negligible heat capacity)containing 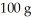 of water at 5.0°C.When equilibrium is reached,how much of the ice will have melted? The specific heat of ice is 2090 J/kg ∙ K,that of water is 4186 J/kg ∙ K,and the latent heat of fusion of water is 33.5 × 104 J/kg.
of water at 5.0°C.When equilibrium is reached,how much of the ice will have melted? The specific heat of ice is 2090 J/kg ∙ K,that of water is 4186 J/kg ∙ K,and the latent heat of fusion of water is 33.5 × 104 J/kg.
A)2.1 g
B)21 g
C)5.2 g
D)52 g
E)4.4 g
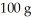 of water at 5.0°C.When equilibrium is reached,how much of the ice will have melted? The specific heat of ice is 2090 J/kg ∙ K,that of water is 4186 J/kg ∙ K,and the latent heat of fusion of water is 33.5 × 104 J/kg.
of water at 5.0°C.When equilibrium is reached,how much of the ice will have melted? The specific heat of ice is 2090 J/kg ∙ K,that of water is 4186 J/kg ∙ K,and the latent heat of fusion of water is 33.5 × 104 J/kg.A)2.1 g
B)21 g
C)5.2 g
D)52 g
E)4.4 g

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
72
A 600-g piece of iron at 100°C is dropped into a calorimeter of negligible specific heat containing 100 g of ice at 0°C and 120 g of water,also at 0°C.What is the final temperature of the system? The specific heat of iron is 448 J/kg ∙ K,the latent heat of fusion of water is 33.5 × 104 J/kg,and the specific heat of water is 4186 J/kg ∙ K.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
73
A person makes iced tea by adding ice to 1.8 kg of hot tea,initially at 80°C.How many kilograms of ice,initially at 0°C,are required to bring the mixture to 10°C? The specific heat of water (and tea)is 4186 J/kg ∙ K,and the latent heat of fusion of ice is 3.34 × 105 J/kg.
A)1.0 kg
B)1.2 kg
C)1.4 kg
D)1.7 kg
A)1.0 kg
B)1.2 kg
C)1.4 kg
D)1.7 kg

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
74
When 50 g of a certain material at 100°C is mixed with 100 g of water at 0°C,the final temperature is 40°C.What is the specific heat of the material? The specific heat of water is 1.00 kcal/kg ∙ C°.
A)0.33 kcal/kg ∙ C°
B)0.75 kcal/kg ∙ C°
C)1.3 kcal/kg ∙ C°
D)7.5 kcal/kg ∙ C°
A)0.33 kcal/kg ∙ C°
B)0.75 kcal/kg ∙ C°
C)1.3 kcal/kg ∙ C°
D)7.5 kcal/kg ∙ C°

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
75
A 400-g block of iron at 400°C is dropped into a calorimeter (of negligible heat capacity)containing 60 g of water at 30°C.How much steam is produced? The latent heat of vaporization of water is 22.6 × 105 J/kg and its specific heat is 4186 J/kg ∙ K.The average specific heat of iron over this temperature range is 560 J/kg ∙ K.
A)22 g
B)33 g
C)42 g
D)54 g
E)59 g
A)22 g
B)33 g
C)42 g
D)54 g
E)59 g

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
76
If 50 g of lead (of specific heat 0.11 kcal/kg ∙ C°)at 100°C is put into 75 g of water (of specific heat 1.0 kcal/kg ∙ C°)at 0°C.What is the final temperature of the mixture?
A)2.0°C
B)6.8°C
C)25°C
D)50°C
A)2.0°C
B)6.8°C
C)25°C
D)50°C

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
77
A 90-g aluminum calorimeter contains 390 g of water at an equilibrium temperature of 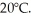 A
A 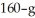 piece of metal,initially at
piece of metal,initially at 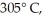 is added to the calorimeter.The final temperature at equilibrium is 32° C.Assume there is no external thermal energy exchange.The specific heat capacities of aluminum and water are 910 J/kg ∙ K (aluminum)and 4190 J/kg ∙ K (water).What is the specific heat of the 160-g piece of metal?
is added to the calorimeter.The final temperature at equilibrium is 32° C.Assume there is no external thermal energy exchange.The specific heat capacities of aluminum and water are 910 J/kg ∙ K (aluminum)and 4190 J/kg ∙ K (water).What is the specific heat of the 160-g piece of metal?
A)470 J/kg ∙ K
B)430 J/kg ∙ K
C)350 J/kg ∙ K
D)310 J/kg ∙ K
E)510 J/kg ∙ K
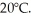 A
A 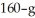 piece of metal,initially at
piece of metal,initially at 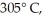 is added to the calorimeter.The final temperature at equilibrium is 32° C.Assume there is no external thermal energy exchange.The specific heat capacities of aluminum and water are 910 J/kg ∙ K (aluminum)and 4190 J/kg ∙ K (water).What is the specific heat of the 160-g piece of metal?
is added to the calorimeter.The final temperature at equilibrium is 32° C.Assume there is no external thermal energy exchange.The specific heat capacities of aluminum and water are 910 J/kg ∙ K (aluminum)and 4190 J/kg ∙ K (water).What is the specific heat of the 160-g piece of metal?A)470 J/kg ∙ K
B)430 J/kg ∙ K
C)350 J/kg ∙ K
D)310 J/kg ∙ K
E)510 J/kg ∙ K

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
78
A piece of iron of mass 0.12 kg is taken from an oven where its temperature is 336°C and quickly placed in an insulated copper can that contains 0.20 kg of water.The copper can has mass 0.50 kg,and it and the water in it are originally at a temperature of 20°C.Calculate the final temperature of the system,assuming no thermal energy is transferred to the surroundings.Use the following specific heats: 4190J/kg ∙ C° (water),470 J/kg ∙ C° (iron),and 390 J/kg ∙ C° (copper).

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
79
A substance has a melting point of 20°C and a heat of fusion of 3.4 × 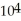 J/kg.The boiling point is
J/kg.The boiling point is 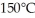 and the heat of vaporization is
and the heat of vaporization is 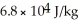 at a pressure of one atmosphere.The specific heats for the solid,liquid,and gaseous phases are 600 J/kg ∙ K (solid),1000 J/kg ∙ K (liquid),and 400 J/kg ∙ K (gaseous).How much thermal energy is required to raise the temperature of
at a pressure of one atmosphere.The specific heats for the solid,liquid,and gaseous phases are 600 J/kg ∙ K (solid),1000 J/kg ∙ K (liquid),and 400 J/kg ∙ K (gaseous).How much thermal energy is required to raise the temperature of 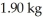 this substance from
this substance from 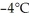 to
to 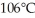 at a pressure of one atmosphere?
at a pressure of one atmosphere?
A)260 kJ
B)190 kJ
C)230 kJ
D)92 kJ
E)320 kJ
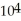 J/kg.The boiling point is
J/kg.The boiling point is 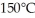 and the heat of vaporization is
and the heat of vaporization is 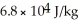 at a pressure of one atmosphere.The specific heats for the solid,liquid,and gaseous phases are 600 J/kg ∙ K (solid),1000 J/kg ∙ K (liquid),and 400 J/kg ∙ K (gaseous).How much thermal energy is required to raise the temperature of
at a pressure of one atmosphere.The specific heats for the solid,liquid,and gaseous phases are 600 J/kg ∙ K (solid),1000 J/kg ∙ K (liquid),and 400 J/kg ∙ K (gaseous).How much thermal energy is required to raise the temperature of 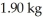 this substance from
this substance from 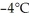 to
to 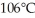 at a pressure of one atmosphere?
at a pressure of one atmosphere?A)260 kJ
B)190 kJ
C)230 kJ
D)92 kJ
E)320 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
80
A lab student drops a 400.0-g piece of metal at 120.0°C into a cup containing 450.0 g of water at 15.0°C.After waiting for a few minutes,the student measures that the final temperature of the system is 40.0°C.What is the specific heat of the metal,assuming that no significant thermal energy is exchanged with the surroundings or the cup? The specific heat of water is 4186 J/kg ∙ K.
A)1470 J/kg ∙ K
B)2830 J/kg ∙ K
C)3420 J/kg ∙ K
D)3780 J/kg ∙ K
E)4280 J/kg ∙ K
A)1470 J/kg ∙ K
B)2830 J/kg ∙ K
C)3420 J/kg ∙ K
D)3780 J/kg ∙ K
E)4280 J/kg ∙ K

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck


