Exam 13: First Law of Thermodynamics
Exam 1: Introduction32 Questions
Exam 2: Kinematics: Motion in One Dimension162 Questions
Exam 3: Newtonian Mechanics54 Questions
Exam 4: Applying Newtons Laws178 Questions
Exam 5: Circular Motion119 Questions
Exam 6: Impulse and Linear Momentum82 Questions
Exam 7: Work and Energy191 Questions
Exam 8: Extended Bodies at Rest59 Questions
Exam 9: Rotational Motion124 Questions
Exam 10: Gases84 Questions
Exam 11: Static Fluids65 Questions
Exam 12: Fluids in Motion30 Questions
Exam 13: First Law of Thermodynamics119 Questions
Exam 14: Second Law of Thermodynamics70 Questions
Exam 15: Electric Charge force and Energy78 Questions
Exam 16: The Electric Field151 Questions
Exam 17: Dc Circuits225 Questions
Exam 18: Magnetism136 Questions
Exam 19: Electromagnetic Induction99 Questions
Exam 20: Vibrational Motion94 Questions
Exam 21: Mechanical Waves155 Questions
Exam 22: Reflection and Refraction36 Questions
Exam 23: Mirrors and Lenses221 Questions
Exam 24: Wave Optics135 Questions
Exam 25: Electromagnetic Waves79 Questions
Exam 26: Special Relativity124 Questions
Exam 27: Quantum Optics100 Questions
Exam 28: Atomic Physics141 Questions
Exam 29: Nuclear Physics155 Questions
Exam 30: Particle Physics21 Questions
Select questions type
A compression at a constant pressure of 200 kPa is performed on 8.00 moles of an ideal monatomic gas.The compression reduces the volume of the gas from to How much work was done on the gas during this process?
Free
(Multiple Choice)
4.7/5  (27)
(27)
Correct Answer:
B
A camper is about to drink his morning coffee.He pours 400 grams of coffee,initially at 75°C into a 250-g aluminum cup,initially at 16°C.What is the equilibrium temperature of the coffee-cup system,assuming no thermal energy exchange with the surroundings? The specific heat of aluminum is 900 J/kg ∙ K,and the specific heat of coffee is essentially the same as that of water,which is 4186 J/kg ∙ K.
Free
(Multiple Choice)
4.8/5  (34)
(34)
Correct Answer:
D
A monatomic ideal gas undergoes an isothermal expansion at 300 K,as the volume increased from to The final pressure is What is the change in the internal (thermal)energy of the gas during this process? (R = 8.31 J/mol ∙ K)
Free
(Multiple Choice)
4.7/5  (33)
(33)
Correct Answer:
A
An ideal gas is compressed isobarically to one-third of its initial volume.The resulting pressure will be
(Multiple Choice)
4.9/5  (25)
(25)
A fluid in an insulated,flexible bottle is heated by a high resistance wire and expands.If of thermal energy is transferred through heating to the system and it - of work is done on it,how much does the internal (thermal)energy of the fluid change?
(Multiple Choice)
4.9/5  (45)
(45)
A person makes iced tea by adding ice to 1.8 kg of hot tea,initially at 80°C.How many kilograms of ice,initially at 0°C,are required to bring the mixture to 10°C? The specific heat of water (and tea)is 4186 J/kg ∙ K,and the latent heat of fusion of ice is 3.34 × 105 J/kg.
(Multiple Choice)
4.8/5  (37)
(37)
A jogger is running outdoors on a cold day when the temperature is -20.0°C.She is breathing at the rate of 25 breaths per minute,and each time she breathes in she inhales 0.00450 m3 of air.How much thermal energy does she lose from breathing during 20.0 minutes of jogging if the air in her lungs is heated to her body temperature of 37.0°C before it is exhaled? The specific heat of air is 1020 J/kg ∙ K and the density of air under typical conditions is 1.29 kg/m3.
(Multiple Choice)
4.9/5  (39)
(39)
Thermal energy through the process of heating is added to a 3.0 kg piece of ice at a rate of How long will it take for the ice at 0.0° C to melt? For water LF = 334,000 J/kg and LV = 2.246 × 106 J/kg.
(Multiple Choice)
4.8/5  (29)
(29)
The temperature of an ideal gas in a sealed rigid 0.20- container is reduced from 360 K to and the final pressure of the gas is How much work is done on the gas during this process? (R = 8.31 J/mol ∙ K)
(Multiple Choice)
4.7/5  (38)
(38)
An architect is interested in estimating the rate of energy loss through heating,ΔQ/Δt,through a sheet of insulating material as a function of the thickness of the sheet.Assuming fixed temperatures on the two faces of the sheet and steady state thermal energy flow,which one of the graphs shown in the figure best represents the rate of thermal energy transfer as a function of the thickness of the insulating sheet? 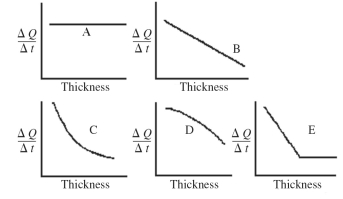
(Multiple Choice)
4.7/5  (34)
(34)
The water flowing over Niagara Falls drops a distance of 50 m.If all the gravitational potential energy of the system water-Earth is converted to thermal energy,by what temperature does the water rise? The specific heat of water is 4186 J/kg ∙ K.
(Multiple Choice)
4.9/5  (40)
(40)
A 40.0-g block of ice at -15.00°C is dropped into a calorimeter (of negligible heat capacity)containing water at 15.00°C.When equilibrium is reached,the final temperature is 8.00°C.How much water did the calorimeter contain initially? The specific heat of ice is 2090 J/kg ∙ K,that of water is 4186 J/kg ∙ K,and the latent heat of fusion of water is 33.5 × 104 J/kg.
(Multiple Choice)
4.8/5  (38)
(38)
Object 1 has three times the specific heat capacity and four times the mass of Object 2.The two objects are heated from the same initial temperature,T0,to the same final temperature Tf.If during this process,if Object 1 absorbs energy through heating Q,the amount of energy absorbed through heating by Object 2 will be
(Multiple Choice)
4.9/5  (43)
(43)
If,with steady state thermal energy flow established,you double the thickness of a wall built from solid uniform material,the rate of thermal energy transfer our of the room for a given temperature difference across the thickness will
(Multiple Choice)
4.8/5  (34)
(34)
It is necessary to determine the specific heat of an unknown object.The mass of the object is It is determined experimentally that it takes to raise the temperature What is the specific heat of the object?
(Multiple Choice)
4.8/5  (33)
(33)
If 50 g of lead (of specific heat 0.11 kcal/kg ∙ C°)at 100°C is put into 75 g of water (of specific heat 1.0 kcal/kg ∙ C°)at 0°C.What is the final temperature of the mixture?
(Multiple Choice)
4.9/5  (37)
(37)
A thermally isolated system is made up of a hot piece of aluminum and a cold piece of copper,with the aluminum and the copper in thermal contact.The specific heat capacity of aluminum is more than double that of copper.Which object will have the greater temperature change during the time the system takes to reach thermal equilibrium?
(Multiple Choice)
4.8/5  (35)
(35)
On a cold day,a piece of metal feels much colder to the touch than a piece of wood.This is due to the difference in which one of the following physical properties of these materials?
(Multiple Choice)
4.9/5  (39)
(39)
How much thermal energy must be removed from 456 g of water at 25.0°C to change it into ice at -10.0°C? The specific heat of ice is 2090 J/kg ∙ K,the latent heat of fusion of water is 33.5 × 104 J/kg,and the specific heat of water is 4186 J/kg ∙ K.
(Multiple Choice)
4.9/5  (24)
(24)
Some properties of a certain glass are listed here: Density 2300 kg/m3
Specific heat capacity 840 J/kg ∙ C°
Coefficient of thermal expansion 8.5 × 10-6 (C°)-1
Thermal conductivity 0.80 W/m ∙ C°
A glass window pane is 2.7 m high,2.4 m wide,and 9.0 mm thick.The temperature at the inner surface of the glass is and at the outer surface 4°C.How much thermal energy is lost each hour through the window?
(Multiple Choice)
4.7/5  (28)
(28)
Showing 1 - 20 of 119
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)