Deck 6: The Shape of Molecules
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/98
Play
Full screen (f)
Deck 6: The Shape of Molecules
1
Which of the following statements is correct about the bent structure?
A)has three bonding electrons pairs with at least one lone pair around the central atom
B)is the shape of the ammonia molecule
C)has its angles around the central atom very close to the 105°
D)All above statements are correct.
A)has three bonding electrons pairs with at least one lone pair around the central atom
B)is the shape of the ammonia molecule
C)has its angles around the central atom very close to the 105°
D)All above statements are correct.
has its angles around the central atom very close to the 105°
2
Dipole dipole forces are an important factor to consider in determining whether or not a substance will dissolve in water (a molecule which has a dipole). Which of the following compounds would you expect to be the least soluble in water?
A)BF3
B)NH3
C)HF
D)H2S
A)BF3
B)NH3
C)HF
D)H2S
BF3
3
When drawing the three-dimensional structure diagram of a molecule the bold line ________.
A)projects out from the page
B)goes back behind the page
C)the bond is in the plane of the page
D)One cannot say unless the formula of the molecule is known.
A)projects out from the page
B)goes back behind the page
C)the bond is in the plane of the page
D)One cannot say unless the formula of the molecule is known.
projects out from the page
4
Which compound listed below has a bond angle of 180° around the central atom?
A)H2O
B)CO2
C)CC14
D)NH3
A)H2O
B)CO2
C)CC14
D)NH3

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
5
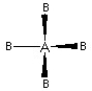
Which of the following pictures best illustrates a tetrahedral shape for the fictional molecule AB4?
A)
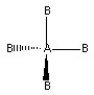
B)
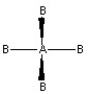
C)
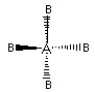
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
6
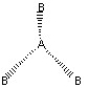
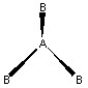
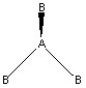
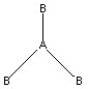
Which of the following pictures best illustrates a planar triangle shape for the fictional molecule AB3 ?
A)
B)
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
7
In which of the three phases of matter would you expect hydrogen bonding between water molecules to be the strongest?
A)solid phase (H2O Os very close together)
B)liquid phase (H2O Os more separated than solid phase)
C)gas phase (H2O Os much farther apart than liquid phase)
D)It is equally strong in all three phases.
A)solid phase (H2O Os very close together)
B)liquid phase (H2O Os more separated than solid phase)
C)gas phase (H2O Os much farther apart than liquid phase)
D)It is equally strong in all three phases.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following statements is correct about the pyramidal structure?
A)is very similar to the tetrahedral structure except that the pair electrons in the pyramidal structure replaces a bond in the tetrahedral one
B)is the shape of the ammonia molecule
C)has its angles around the central atom very close to the 109°
D)All above statements are correct.
A)is very similar to the tetrahedral structure except that the pair electrons in the pyramidal structure replaces a bond in the tetrahedral one
B)is the shape of the ammonia molecule
C)has its angles around the central atom very close to the 109°
D)All above statements are correct.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
9
Dipole dipole forces are an important factor to consider in determining whether or not a substance will dissolve in water (a molecule which has a dipole). Which of the following compounds would you expect to be the most soluble in water?
A)CCl4
B)NH3
C)N2
D)CO2
A)CCl4
B)NH3
C)N2
D)CO2

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following compounds contains bonds that are the most polar (greatest electronegativity difference between hydrogen and the central atom)?
A)H2O
B)H2S
C)H2Se
D)H2Te
A)H2O
B)H2S
C)H2Se
D)H2Te

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
11
In a molecule having three pairs of electrons around a central atom and a planar triangle shape, how many lone pairs of electrons does the central atom have?
A)1
B)0
C)2
D)3
A)1
B)0
C)2
D)3

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
12
In a molecule having four pairs of electrons around the central atom and a pyramidal shape, how many lone pairs of electrons does the central atom have?
A)2
B)1
C)4
D)3
A)2
B)1
C)4
D)3

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
13
Which compound listed below would you expect to have the greatest H-C-H bond angle?
A)CH2ClF
B)CH2 CI2
C)CH4
D)CH2O
A)CH2ClF
B)CH2 CI2
C)CH4
D)CH2O

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following would you expect to be the most polar molecule?
A)H2S
B)H2Se
C)H2Te
D)H2O
A)H2S
B)H2Se
C)H2Te
D)H2O

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
15
In a molecule having four pairs of electrons around the central atom and a pyramidal shape, how many bonding pairs of electrons does the central atom have?
A)2
B)1
C)4
D)3
A)2
B)1
C)4
D)3

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
16
When drawing the three-dimensional structure diagram of a molecule the dotted line ________.
A)projects out from the page
B)goes back behind the page
C)the bond is in the plane of the page
D)One cannot say unless the formula of the molecule is known.
A)projects out from the page
B)goes back behind the page
C)the bond is in the plane of the page
D)One cannot say unless the formula of the molecule is known.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
17
When drawing the three-dimensional structure diagram of a molecule the solid (not bold or dotted)line ________.
A)projects out from the page
B)goes back behind the page
C)the bond is in the plane of the page
D)One cannot say unless the formula of the molecule is known.
A)projects out from the page
B)goes back behind the page
C)the bond is in the plane of the page
D)One cannot say unless the formula of the molecule is known.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is linear?
A)hydrogen chloride
B)ethane, C2H6
C)ethene, C2H4
D)ammonia
A)hydrogen chloride
B)ethane, C2H6
C)ethene, C2H4
D)ammonia

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following molecules is not linear?
A)hydrogen cyanide
B)beryllium chloride
C)boron trifluoride
D)acetylene, C2H2
A)hydrogen cyanide
B)beryllium chloride
C)boron trifluoride
D)acetylene, C2H2

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
20
In a molecule having four pairs of electrons around the center atom and a bent shape, how many bonding pairs does the central atom have?
A)2
B)1
C)4
D)3
A)2
B)1
C)4
D)3

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following bonds will have the largest difference in electronegativity?
A)HBr
B)NaF
C)H2
D)HS-
A)HBr
B)NaF
C)H2
D)HS-

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is tetrahedral?
A)sulfur trioxide
B)sulfur dioxide
C)sulfur dichloride
D)ammonium ion
A)sulfur trioxide
B)sulfur dioxide
C)sulfur dichloride
D)ammonium ion

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following will be the least soluble in water?
A)CH3OH
B)CH4
C)NH3
D)CH3CH2OH
A)CH3OH
B)CH4
C)NH3
D)CH3CH2OH

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following multiple-bond compounds is polar?
A)N2
B)O2
C)SOCl2
D)CO2
A)N2
B)O2
C)SOCl2
D)CO2

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
25
The angle around boron in boron trifluoride is ________.
A)60°
B)90°
C)109°
D)120°
A)60°
B)90°
C)109°
D)120°

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
26
The angle around nitrogen in the ammonium ion is ________.
A)60°
B)90°
C)109°
D)120°
A)60°
B)90°
C)109°
D)120°

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is non-polar?
A)acetylene, C2H2
B)ethene, C2H4
C)methane
D)All of the above are non-polar.
A)acetylene, C2H2
B)ethene, C2H4
C)methane
D)All of the above are non-polar.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following is pyramidal and has an angle close to 109° around the central atom?
A)sulfur dioxide
B)boron trifluoride
C)ammonia
D)both A and C
A)sulfur dioxide
B)boron trifluoride
C)ammonia
D)both A and C

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
29
The angle around carbon in hydrogen cyanide is ________.
A)105°
B)109°
C)120°
D)180°
A)105°
B)109°
C)120°
D)180°

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the double-bond bearing compounds is polar?
A)SO3
B)CO2
C)CS2
D)H2CO
A)SO3
B)CO2
C)CS2
D)H2CO

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following sulfur-containing species is not polar?
A)SO3
B)H2S
C)HS-
D)SOCl2
A)SO3
B)H2S
C)HS-
D)SOCl2

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is not tetrahedral?
A)carbon tetrachloride
B)chloroform, CHCl3
C)ethane, C2H6
D)boron trifluoride
A)carbon tetrachloride
B)chloroform, CHCl3
C)ethane, C2H6
D)boron trifluoride

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following chlorine-containing species is not polar?
A)CHCl3
B)CH2Cl2
C)HCl
D)CCl4
A)CHCl3
B)CH2Cl2
C)HCl
D)CCl4

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following multiple-bond compounds is pure covalent?
A)H2CO
B)Cl2SO
C)O2
D)CO
A)H2CO
B)Cl2SO
C)O2
D)CO

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following nitrogen-containing species is least polar?
A)NH3
B)CH3 - N = N - CH3
C)(CH3 CH2)3N
D)(CH3)3N
A)NH3
B)CH3 - N = N - CH3
C)(CH3 CH2)3N
D)(CH3)3N

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following bonds will have the smallest difference in electronegativity?
A)HBr
B)NaF
C)H2
D)HS-
A)HBr
B)NaF
C)H2
D)HS-

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following double-bond bearing compounds is non-polar?
A)SO2
B)CH2O
C)CS2
D)SOCl2
A)SO2
B)CH2O
C)CS2
D)SOCl2

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is bent?
A)water
B)sulfur dioxide
C)carbon dioxide
D)both A and B
A)water
B)sulfur dioxide
C)carbon dioxide
D)both A and B

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following is bent and has an angle close to 118° around the central atom?
A)sulfur dioxide
B)boron trifluoride
C)ammonia
D)both A and C
A)sulfur dioxide
B)boron trifluoride
C)ammonia
D)both A and C

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following will be affected by the presence of an electric field?
A)H2
B)O2
C)HCl
D)N2
A)H2
B)O2
C)HCl
D)N2

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
41
Carbon tetrachloride contains polar bonds and is a polar molecule.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
42
What is the steric number of the central atom in boron trichloride?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
43
What is the steric number of the central atom in carbon disulfide?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
44
What is the molecular shape of CC14?
A)tetrahedral
B)pyramidal
C)linear
D)bent
A)tetrahedral
B)pyramidal
C)linear
D)bent

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
45
Carbon dioxide contains polar bonds and is a nonpolar molecule.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
46
The ball-and-stick model emphasizes the bonding pattern.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
47
What is the molecular shape of CH2O?
A)tetrahedral
B)bent
C)trigonal planar
D)pyramidal
A)tetrahedral
B)bent
C)trigonal planar
D)pyramidal

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
48
Bonds are regions of negative charge and therefore repel each other.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
49
Any molecule of the form AX3 is tetrahedral.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
50
Carbon tetrachloride is polar.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
51
All of the following SO3, CO2, CS2 are non-polar.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
52
In the VSEPR model the multiple bond is treated as a single electron group, just the way a single bond is treated.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following compounds would be soluble in a nonpolar solvent?
A)H2S
B)NH3
C)CC14
D)SO2
A)H2S
B)NH3
C)CC14
D)SO2

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
54
When drawing the three-dimensional structure diagram of a molecule the bold line goes back behind the page.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
55
All of the following SO2, HCl, CO2 are linear.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
56
What is the steric number of the central atom in CH4?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
57
The angle around the central atom in the pyramidal structure is very close to that of the tetrahedral one.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
58
All of the following O2, HI, CO2 are linear.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
59
What is the molecular shape of NO2-
A)tetrahedral
B)pyramidal
C)linear
D)bent
A)tetrahedral
B)pyramidal
C)linear
D)bent

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
60
What is the molecular shape of H2S?
A)tetrahedral
B)pyramidal
C)linear
D)bent
A)tetrahedral
B)pyramidal
C)linear
D)bent

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
61
Judge whether or not the molecule or polyatomic ion shown is polar or non-polar. Draw a dipole vector to indicate the direction of any net polarity.
PH3
PH3

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
62
The bond angle in ammonia is 109°.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
63
Judge whether or not the molecule or polyatomic ion shown is polar or non-polar. Draw a dipole vector to indicate the direction of any net polarity.
O3 (ozone)
O3 (ozone)

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
64
Sulfur dioxide is linear.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
65
Judge whether or not the molecule or polyatomic ion shown is polar or non-polar. Draw a dipole vector to indicate the direction of any net polarity.
H2S
H2S

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
66
Judge whether or not the molecule or polyatomic ion shown is polar or non-polar. Draw a dipole vector to indicate the direction of any net polarity.
CN-
CN-

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
67
A polar covalent compound is unaffected by the presence of an electric field.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
68
When naming the shape of a molecule count the lone pairs.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
69
Judge whether or not the molecule or polyatomic ion shown is polar or non-polar. Draw a dipole vector to indicate the direction of any net polarity.
SO2
SO2

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
70
One can say with certainty that the pyramidal structure is the tetrahedral arrangement minus a bond but with a lone pair.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
71
The difference in electronegativity between H and in HF is higher than that between H and Cl in HCl.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
72
Judge whether or not the molecule or polyatomic ion shown is polar or non-polar. Draw a dipole vector to indicate the direction of any net polarity.
SCN-
SCN-

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
73
Judge whether or not the molecule or polyatomic ion shown is polar or non-polar. Draw a dipole vector to indicate the direction of any net polarity.
CHC13
CHC13

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
74
Compounds with polar bonds may be nonpolar.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
75
Judge whether or not the molecule or polyatomic ion shown is polar or non-polar. Draw a dipole vector to indicate the direction of any net polarity.
CS2
CS2

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
76
Judge whether or not the molecule or polyatomic ion shown is polar or non-polar. Draw a dipole vector to indicate the direction of any net polarity.
NO3-
NO3-

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
77
The bond angle in carbon tetrachloride is 109°.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
78
Judge whether or not the molecule or polyatomic ion shown is polar or non-polar. Draw a dipole vector to indicate the direction of any net polarity.
PO43-
PO43-

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
79
Judge whether or not the molecule or polyatomic ion shown is polar or non-polar. Draw a dipole vector to indicate the direction of any net polarity.
CO32-
CO32-

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
80
When determining the shape of a molecule, count all electron groups around the central atom.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck


