Exam 6: The Shape of Molecules
Exam 1: What Is Chemistry51 Questions
Exam 2: The Numerical Side of Chemistry147 Questions
Exam 3: The Evolution of Atomic Theory92 Questions
Exam 4: The Modern Model of the Atom97 Questions
Exam 5: Chemical Bonding and Nomenclature245 Questions
Exam 6: The Shape of Molecules92 Questions
Exam 7: Intermolecular Forces and the Phases of Matter67 Questions
Exam 8: Chemical Reactions75 Questions
Exam 9: Stoichiometry and the Mole191 Questions
Exam 10: Electron Transfer in Chemical Reactions87 Questions
Exam 11: What If There Were No Intermolecular Forces the Ideal Gas80 Questions
Exam 12: Solutions92 Questions
Exam 13: When Reactants Turn Into Products79 Questions
Exam 14: Chemical Equilibrium94 Questions
Exam 15: Electrolytes, Acids, and Bases94 Questions
Exam 16: Nuclear Chemistry84 Questions
Exam 17: The Chemistry of Carbon71 Questions
Exam 18: Synthetic and Biological Polymers47 Questions
Select questions type
When drawing the three-dimensional structure diagram of a molecule the bold line ________.
Free
(Multiple Choice)
4.8/5  (41)
(41)
Correct Answer:
A
What is the steric number of the central atom in boron trichloride?
Free
(Multiple Choice)
4.9/5  (38)
(38)
Correct Answer:
C
Judge whether or not the molecule or polyatomic ion shown is polar or non-polar. Draw a dipole vector to indicate the direction of any net polarity.
-NO+
Free
(Short Answer)
4.8/5  (35)
(35)
Correct Answer:
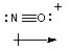
Judge whether or not the molecule or polyatomic ion shown is polar or non-polar. Draw a dipole vector to indicate the direction of any net polarity.
-SO3
(Short Answer)
4.8/5  (33)
(33)
Which of the following bonds will have the smallest difference in electronegativity?
(Multiple Choice)
4.8/5  (30)
(30)
Dipole dipole forces are an important factor to consider in determining whether or not a substance will dissolve in water (a molecule which has a dipole). Which of the following compounds would you expect to be the least soluble in water?
(Multiple Choice)
4.8/5  (39)
(39)
Judge whether or not the molecule or polyatomic ion shown is polar or non-polar. Draw a dipole vector to indicate the direction of any net polarity.
-CSe2
(Short Answer)
4.8/5  (32)
(32)
Which of the following chlorine-containing species is not polar?
(Multiple Choice)
4.8/5  (40)
(40)
Judge whether or not the molecule or polyatomic ion shown is polar or non-polar. Draw a dipole vector to indicate the direction of any net polarity.
-O3 (ozone)
(Short Answer)
4.7/5  (41)
(41)
Which of the following would you expect to be the most polar molecule?
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following is bent and has an angle close to 118° around the central atom?
(Multiple Choice)
4.7/5  (41)
(41)
In a molecule having four pairs of electrons around the central atom and a pyramidal shape, how many bonding pairs of electrons does the central atom have?
(Multiple Choice)
4.8/5  (41)
(41)
Match each molecule in the questions with an angle around the central atom that appears in the list below.
Correct Answer:
Premises:
Responses:
(Matching)
4.8/5  (23)
(23)
Showing 1 - 20 of 92
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)