Deck 6: Principles of Chemical Reactivity: Energy and Chemical Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/74
Play
Full screen (f)
Deck 6: Principles of Chemical Reactivity: Energy and Chemical Reactions
1
The energy stored in a fuel is called _____.
A) heat
B) internal energy
C) temperature
D) kinetic energy
E) potential energy
A) heat
B) internal energy
C) temperature
D) kinetic energy
E) potential energy
potential energy
2
If 50.0 g of benzene,C6H6,at 25.0 C absorbs 2.71 kJ of energy in the form of heat,what is the final temperature of the benzene? The specific heat capacity of benzene is 1.72 J/g.K.
A) 25.0 C
B) 31.5 C
C) 56.5 C
D) 32.3 C
E) 57.3 C
A) 25.0 C
B) 31.5 C
C) 56.5 C
D) 32.3 C
E) 57.3 C
56.5 C
3
Exactly 212.2 J will raise the temperature of 10.0 g of a metal from 25.0 °C to 60.0 °C.What is the specific heat capacity of the metal?
A) 0.606 J/(g·°C)
B) 1.65 J/(g·°C)
C) 14.5 J/(g·°C)
D) 50.8 J/(g·°C)
E) none of these
A) 0.606 J/(g·°C)
B) 1.65 J/(g·°C)
C) 14.5 J/(g·°C)
D) 50.8 J/(g·°C)
E) none of these
0.606 J/(g·°C)
4
It is relatively easy to change the temperature of a substance that
A) is very massive.
B) is an insulator.
C) has a high specific heat capacity.
D) has a low specific heat capacity.
E) is brittle.
A) is very massive.
B) is an insulator.
C) has a high specific heat capacity.
D) has a low specific heat capacity.
E) is brittle.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
5
A 100 g sample of each of the following metals is heated from 35 C to 45 C.Which metal absorbs the greatest amount of heat energy?

A) magnesium
B) lead
C) mercury
D) silver
E) copper

A) magnesium
B) lead
C) mercury
D) silver
E) copper

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
6
Which one of the following statements is INCORRECT?
A) Energy is neither created nor destroyed in chemical reactions.
B) Kinetic energy is the energy that results from an object's position.
C) Exothermic processes transfer heat from the system into the surroundings.
D) Increasing the thermal energy of a gas increases the motion of its atoms.
E) Energy is the capacity to do work.
A) Energy is neither created nor destroyed in chemical reactions.
B) Kinetic energy is the energy that results from an object's position.
C) Exothermic processes transfer heat from the system into the surroundings.
D) Increasing the thermal energy of a gas increases the motion of its atoms.
E) Energy is the capacity to do work.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
7
Specific heat capacity is
A) the quantity of heat needed to change the temperature of 1.00 g of a substance by 1 K.
B) the quantity of heat needed to change the temperature of 1.00 g of a substance by 4.184 K.
C) the capacity of a substance to gain or lose a 1.00 J of energy in the form of heat.
D) the temperature change undergone when 1.00 g of a substance absorbs 4.184 J.
E) the maximum amount of energy in the form of heat that 1.00 g of a substance may absorb without decomposing.
A) the quantity of heat needed to change the temperature of 1.00 g of a substance by 1 K.
B) the quantity of heat needed to change the temperature of 1.00 g of a substance by 4.184 K.
C) the capacity of a substance to gain or lose a 1.00 J of energy in the form of heat.
D) the temperature change undergone when 1.00 g of a substance absorbs 4.184 J.
E) the maximum amount of energy in the form of heat that 1.00 g of a substance may absorb without decomposing.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
8
The specific heat capacity of copper is 0.384 J/g. C.What is the molar specific heat capacity of this substance? The molar mass of copper is 63.54 g/mol.
A) 24.4 J/mol. C
B) 0.00604 J/mol. C
C) 165 J/mol. C
D) 0.384 J/mol. C
E) 2.60 J/mol. C
A) 24.4 J/mol. C
B) 0.00604 J/mol. C
C) 165 J/mol. C
D) 0.384 J/mol. C
E) 2.60 J/mol. C

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
9
A 170.0-g sample of metal at 79.00°C is added to 170.0 g of H2O(l)at 14.00°C in an insulated container.The temperature rises to 16.19°C.Neglecting the heat capacity of the container,what is the specific heat capacity of the metal? The specific heat capacity of H2O(l)is 4.18 J/(g·°C).
A) 4.18 J/(g·°C)
B) 120 J/(g·°C)
C) 0.146 J/(g·°C)
D) -0.146 J/(g·°C)
E) 28.6 J/(g·°C)
A) 4.18 J/(g·°C)
B) 120 J/(g·°C)
C) 0.146 J/(g·°C)
D) -0.146 J/(g·°C)
E) 28.6 J/(g·°C)

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
10
Which of these physical changes would require the release of energy?
A) condensing a gas
B) boiling a liquid
C) melting a solid
D) all of these
E) none of these
A) condensing a gas
B) boiling a liquid
C) melting a solid
D) all of these
E) none of these

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following processes is/are exothermic?
1)the reaction of butane with oxygen
2)the melting of gold
3)cooling copper from 225 C to 65 C
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2,and 3
1)the reaction of butane with oxygen
2)the melting of gold
3)cooling copper from 225 C to 65 C
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2,and 3

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
12
A hot piece of iron is dropped into a beaker containing colder water.Which of the following statements is/are CORRECT?
1)Energy is transferred as heat from the iron to the water.
2)Thermal equilibrium is attained when the iron and the water reach the same temperature.
3)Thermal energy from the iron is converted to electrostatic energy in the water.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)Energy is transferred as heat from the iron to the water.
2)Thermal equilibrium is attained when the iron and the water reach the same temperature.
3)Thermal energy from the iron is converted to electrostatic energy in the water.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
13
How much energy is gained by nickel when 18.7 g of nickel is warmed from 20.5 °C to 79.8 °C? The specific heat capacity of nickel is 0.443 J/(g·°C).
A) 1.70 102 J
B) 35.35 J
C) 26.27 J
D) 4.91 102 J
E) 6.61 102 J
A) 1.70 102 J
B) 35.35 J
C) 26.27 J
D) 4.91 102 J
E) 6.61 102 J

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
14
If 35.0 g H2O at 22.7 C is combined with 65.0 g H2O at 87.5 C,what is the final temperature of the mixture? The specific heat capacity of water is 4.184 J/g.K.
A) 25.1 C
B) 45.4 C
C) 50.8 C
D) 64.8 C
E) 48.9 C
A) 25.1 C
B) 45.4 C
C) 50.8 C
D) 64.8 C
E) 48.9 C

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following statements is/are CORRECT?
1)A system is defined as an object or collection of objects being studied.
2)Surroundings are defined as the entire universe,including the system.
3)In an endothermic reaction,heat is transferred from the system to the surroundings.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2,and 3
1)A system is defined as an object or collection of objects being studied.
2)Surroundings are defined as the entire universe,including the system.
3)In an endothermic reaction,heat is transferred from the system to the surroundings.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2,and 3

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
16
Many homes are heated using natural gas.The combustion of natural gas converts
A) chemical potential energy to thermal energy.
B) thermal energy to mechanical energy.
C) mechanical energy to chemical potential.
D) electrostatic energy to mechanical energy.
E) gravitational energy to acoustic energy.
A) chemical potential energy to thermal energy.
B) thermal energy to mechanical energy.
C) mechanical energy to chemical potential.
D) electrostatic energy to mechanical energy.
E) gravitational energy to acoustic energy.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following processes is/are endothermic?
1)the combustion of hydrogen
2)the condensation of water
3)the evaporation of isopropyl alcohol
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2,and 3
1)the combustion of hydrogen
2)the condensation of water
3)the evaporation of isopropyl alcohol
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2,and 3

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is an endothermic process?
A) work is done by the system on the surroundings
B) heat energy flows from the system to the surroundings
C) work is done on the system by the surroundings
D) heat energy is evolved by the system
E) none of the above
A) work is done by the system on the surroundings
B) heat energy flows from the system to the surroundings
C) work is done on the system by the surroundings
D) heat energy is evolved by the system
E) none of the above

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
19
Heat capacity is defined as
A) the amount of heat required to raise the temperature of 1 gram of substance by 1 K.
B) the amount of heat required to raise the temperature of a substance by 1 K.
C) the amount of heat required to vaporize a solid or liquid.
D) the maximum amount of heat that a substance may absorb without decomposing.
E) 4.18 cal/g.K.
A) the amount of heat required to raise the temperature of 1 gram of substance by 1 K.
B) the amount of heat required to raise the temperature of a substance by 1 K.
C) the amount of heat required to vaporize a solid or liquid.
D) the maximum amount of heat that a substance may absorb without decomposing.
E) 4.18 cal/g.K.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following statements is/are CORRECT?
1)Specific heat capacity is a positive value for liquids and solids and a negative value for gases.
2)The larger the heat capacity of an object,the more thermal energy it can store.
3)When heat is transferred from the surroundings to the system,q is negative.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)Specific heat capacity is a positive value for liquids and solids and a negative value for gases.
2)The larger the heat capacity of an object,the more thermal energy it can store.
3)When heat is transferred from the surroundings to the system,q is negative.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
21
The heat of vaporization of benzene,C6H6,is 30.7 kJ/mol at its boiling point of 80.1 C.How much energy in the form of heat is required to vaporize 102 g benzene at its boiling point?
A) 0.302 kJ
B) 23.6 kJ
C) 24.2 kJ
D) 40.1 kJ
E) 3.14 103 kJ
A) 0.302 kJ
B) 23.6 kJ
C) 24.2 kJ
D) 40.1 kJ
E) 3.14 103 kJ

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
22
Given the thermochemical equation 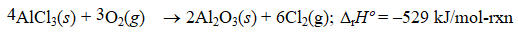
Find rHº for the following reaction.
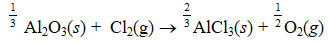
A) (+88.2 kJ/mol-rxn)
B) (+264.5 kJ/mol-rxn)
C) ( +529.0 kJ/mol-rxn)
D) (-176.3 kJ/mol-rxn)
E) (-176.3 kJ/mol-rxn).
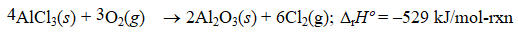
Find rHº for the following reaction.
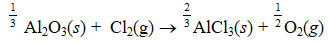
A) (+88.2 kJ/mol-rxn)
B) (+264.5 kJ/mol-rxn)
C) ( +529.0 kJ/mol-rxn)
D) (-176.3 kJ/mol-rxn)
E) (-176.3 kJ/mol-rxn).

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
23
What quantity,in moles,of oxygen is consumed when 369.3 kJ of energy is evolved from the combustion of a mixture of H2(g)and O2(g)?
H2(g)+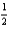
O2(g) H2O(l); rH° = -285.8 kJ/mol-rxn
A) 0.6461 mol
B) 1.292 mol
C) 0.3869 mol
D) 1.146 mol
E) 0.1461 mol
H2(g)+
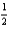
O2(g) H2O(l); rH° = -285.8 kJ/mol-rxn
A) 0.6461 mol
B) 1.292 mol
C) 0.3869 mol
D) 1.146 mol
E) 0.1461 mol

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
24
What is the change in internal energy of the system ( U)if 7 kJ of heat energy is evolved by the system and 99 kJ of work is done on the system for a certain process?
A) 92 kJ
B) -106 kJ
C) -7 kJ
D) -92 kJ
E) 106 kJ
A) 92 kJ
B) -106 kJ
C) -7 kJ
D) -92 kJ
E) 106 kJ

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
25
What is the minimum mass of ice at 0.0 C that must be added to 1.00 kg of water to cool the water from 28.0 C to 12.0 C? (Heat of fusion = 333 J/g; specific heat capacities: ice = 2.06 J/g.K,liquid water = 4.184 J/g.K)
A) 175 g
B) 201 g
C) 244 g
D) 299 g
E) 1140 g
A) 175 g
B) 201 g
C) 244 g
D) 299 g
E) 1140 g

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
26
How much energy is needed to convert 57.5 grams of ice at 0.00°C to liquid water at 75.0°C?
Specific heat capacity (ice)= 2.10 J/g°C
Specific heat capacity (liquid water)= 4.18 J/g°C
Heat of fusion = 333 J/g
Heat of vaporization = 2258 J/g
A) 18.0 kJ
B) 2.06 kJ
C) 28.2 kJ
D) 37.2 kJ
E) 148 kJ
Specific heat capacity (ice)= 2.10 J/g°C
Specific heat capacity (liquid water)= 4.18 J/g°C
Heat of fusion = 333 J/g
Heat of vaporization = 2258 J/g
A) 18.0 kJ
B) 2.06 kJ
C) 28.2 kJ
D) 37.2 kJ
E) 148 kJ

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
27
If 46.1 g Cu at 11.6 C is placed in 85.0 g H2O at 72.4 C,what is the final temperature of the mixture? The specific heat capacities of copper and water are 0.385 J/g.K and 4.184 J/g.K,respectively.
A) 71.2 C
B) 63.6 C
C) 51.0 C
D) 42.0 C
E) 69.5 C
A) 71.2 C
B) 63.6 C
C) 51.0 C
D) 42.0 C
E) 69.5 C

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
28
When 66.0 g of an unknown metal at 28.5 C is placed in 83.0 g H2O at 78.5 C,the water temperature decreases to 75.9 C.What is the specific heat capacity of the metal? The specific heat capacity of water is 4.184 J/g.K.
A) 0.055 J/g.K
B) 0.29 J/g.K
C) 0.69 J/g.K
D) 0.18 J/g.K
E) 2.6 J/g.0K
A) 0.055 J/g.K
B) 0.29 J/g.K
C) 0.69 J/g.K
D) 0.18 J/g.K
E) 2.6 J/g.0K

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
29
A 41.3-g piece of nickel (s = 0.443 J/(g·°C)),initially at 255.8°C,is added to 144.8 g of a liquid,initially at 23.1°C,in an insulated container.The final temperature of the metal-liquid mixture at equilibrium is 35.4°C.What is the identity of the liquid? Neglect the heat capacity of the container.
A) acetone (s = 2.15 J/(g·°C))
B) hexane (s = 2.27 J/(g·°C))
C) water (s = 4.18 J/(g·°C))
D) methanol (s = 2.53 J/(g·°C))
E) ethanol (s = 2.43 J/(g·°C))
A) acetone (s = 2.15 J/(g·°C))
B) hexane (s = 2.27 J/(g·°C))
C) water (s = 4.18 J/(g·°C))
D) methanol (s = 2.53 J/(g·°C))
E) ethanol (s = 2.43 J/(g·°C))

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
30
Calculate U of a gas for a process in which the gas absorbs 19 J of heat and does 49 J of work by expanding.
A) 30 J
B) 68 J
C) -68 J
D) 0,because U is a state function
E) -30 J
A) 30 J
B) 68 J
C) -68 J
D) 0,because U is a state function
E) -30 J

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
31
The thermochemical equation for the combustion of methanol is shown below.
CH3OH(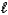 )+ 3/2 O2(g) CO2(g)+ 2 H 2O(g)
)+ 3/2 O2(g) CO2(g)+ 2 H 2O(g)
rH = -638.7 kJ/mol-rxn
What is the enthalpy change for the combustion of 8.59 g CH3OH?
A) -171 kJ
B) -19.9 kJ
C) -2.38 103 kJ
D) -5.49 103 kJ
E) -1.76 106 kJ
CH3OH(
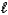 )+ 3/2 O2(g) CO2(g)+ 2 H 2O(g)
)+ 3/2 O2(g) CO2(g)+ 2 H 2O(g)rH = -638.7 kJ/mol-rxn
What is the enthalpy change for the combustion of 8.59 g CH3OH?
A) -171 kJ
B) -19.9 kJ
C) -2.38 103 kJ
D) -5.49 103 kJ
E) -1.76 106 kJ

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following statements is/are CORRECT?
1)If a reaction occurs at constant pressure,q = H.
2)The change in energy for a system is defined as the sum of the energies transferred as heat and work (i.e., U = q + w).
3)If a reaction occurs at constant volume,q = w
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)If a reaction occurs at constant pressure,q = H.
2)The change in energy for a system is defined as the sum of the energies transferred as heat and work (i.e., U = q + w).
3)If a reaction occurs at constant volume,q = w
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
33
Calculate the energy in the form of heat (in kJ)required to convert 325 grams of liquid water at 20.0 C to steam at 115 C.Assume that no energy in the form of heat is transferred to the environment.(Heat of fusion = 333 J/g; heat of vaporization = 2256 J/g; specific heat capacities: liquid water = 4.184 J/g.K,steam = 1.92 J/g.K)
A) 129 kJ
B) 121 kJ
C) 851 kJ
D) 914 kJ
E) 735 kJ
A) 129 kJ
B) 121 kJ
C) 851 kJ
D) 914 kJ
E) 735 kJ

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following processes will result in the lowest final temperature of the metal-water mixture at when thermal equilibrium is reached? The specific heat capacity of nickel is 0.443 J/(g·°C).The specific heat capacity of water is 4.184 J/(g·°C).
A) the addition of 100 g of nickel at 95°C to 80 mL of water at 25°C in an insulated container
B) the addition of 100 g of nickel at 95°C to 100 mL of water at 25°C in an insulated container
C) the addition of 100 g of nickel at 95°C to 40 mL of water at 25°C in an insulated container
D) the addition of 100 g of nickel at 95°C to 20 mL of water at 25°C in an insulated container
E) the addition of 100 g of nickel at 95°C to 60 mL of water at 25°C in an insulated container
A) the addition of 100 g of nickel at 95°C to 80 mL of water at 25°C in an insulated container
B) the addition of 100 g of nickel at 95°C to 100 mL of water at 25°C in an insulated container
C) the addition of 100 g of nickel at 95°C to 40 mL of water at 25°C in an insulated container
D) the addition of 100 g of nickel at 95°C to 20 mL of water at 25°C in an insulated container
E) the addition of 100 g of nickel at 95°C to 60 mL of water at 25°C in an insulated container

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
35
The thermochemical equation for the combustion of butane is shown below.C4H10(g)+ 13/2 O2(g) 4 CO2(g)+ 5 H2O( 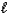 )
)
rH = -2877 kJ/mol-rxn
What is the enthalpy change for the following reaction?
8 CO2(g)+ 10 H2O( 11ea8937_ab5c_9838_a16d_1fd5f2335456_TB4499_11 ) 2 C4H10(g)+ 13 O2(g)
A) +1439 kJ/mol-rxn
B) +2877 kJ/mol-rxn
C) -5754 kJ/mol-rxn
D) -2877 kJ/mol-rxn
E) +5754 kJ/mol-rxn
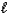 )
)rH = -2877 kJ/mol-rxn
What is the enthalpy change for the following reaction?
8 CO2(g)+ 10 H2O( 11ea8937_ab5c_9838_a16d_1fd5f2335456_TB4499_11 ) 2 C4H10(g)+ 13 O2(g)
A) +1439 kJ/mol-rxn
B) +2877 kJ/mol-rxn
C) -5754 kJ/mol-rxn
D) -2877 kJ/mol-rxn
E) +5754 kJ/mol-rxn

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
36
One statement of the first law of thermodynamics is that
A) the amount of work done on a system is dependent of the pathway.
B) the total work done on a system must equal the heat absorbed by the system.
C) the total work done on a system is equal in magnitude,but opposite in sign of the heat absorbed by the system.
D) the total energy change for a system is equal to the sum of the heat transferred to or from the system and the work done by or on the system.
E) in any chemical process the heat flow must equal the change in enthalpy.
A) the amount of work done on a system is dependent of the pathway.
B) the total work done on a system must equal the heat absorbed by the system.
C) the total work done on a system is equal in magnitude,but opposite in sign of the heat absorbed by the system.
D) the total energy change for a system is equal to the sum of the heat transferred to or from the system and the work done by or on the system.
E) in any chemical process the heat flow must equal the change in enthalpy.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
37
If q = 98 kJ and w = 4 kJ for a certain process,that process
A) requires a catalyst.
B) is endothermic.
C) occurs slowly.
D) is exothermic.
E) cannot occur.
A) requires a catalyst.
B) is endothermic.
C) occurs slowly.
D) is exothermic.
E) cannot occur.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following thermodynamic quantities are state functions: heat (q),work (w),enthalpy change ( H),and/or internal energy change ( U)?
A) q only
B) w only
C) ( H) only
D) ( U) only
E) ( H) and ( U)
A) q only
B) w only
C) ( H) only
D) ( U) only
E) ( H) and ( U)

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
39
At constant pressure and 25 C,what is rH for the following reaction
2C2H6(g)+ 7O2(g) 4CO2(g)+ H2O(l)
If the complete consumption of 89.4 g of C2H6 liberates -4638 kJ of heat energy?
A) -3120 kJ/mol-rxn
B) -1560 kJ/mol-rxn
C) -27600 kJ/mol-rxn
D) -13800 kJ/mol-rxn
E) -787 kJ/mol-rxn
2C2H6(g)+ 7O2(g) 4CO2(g)+ H2O(l)
If the complete consumption of 89.4 g of C2H6 liberates -4638 kJ of heat energy?
A) -3120 kJ/mol-rxn
B) -1560 kJ/mol-rxn
C) -27600 kJ/mol-rxn
D) -13800 kJ/mol-rxn
E) -787 kJ/mol-rxn

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
40
Calculate the energy in the form of heat (in kJ)required to change 71.8 g of liquid water at 25.7 C to ice at -16.1 C.Assume that no energy in the form of heat is transferred to the environment.(Heat of fusion = 333 J/g; heat of vaporization = 2256 J/g; specific heat capacities: ice = 2.06 J/g.K,liquid water = 4.184 J/g.K)
A) -12.6 kJ
B) -7.7 kJ
C) -34.0kJ
D) -31.6 kJ
E) -10.1 kJ
A) -12.6 kJ
B) -7.7 kJ
C) -34.0kJ
D) -31.6 kJ
E) -10.1 kJ

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following has a standard enthalpy of formation value of zero at 25°C?
A) I(g)
B) I2(l)
C) I2(s)
D) I(s)
E) I2(g)
A) I(g)
B) I2(l)
C) I2(s)
D) I(s)
E) I2(g)

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
42
The overall chemical equation resulting from the sum of the following three steps is
2C(s)+ 2H2O(g) 2CO(g)+ 2H2(g)
CO(g)+ H2O(g) CO2(g)+ H2(g)
CO(g)+ 3H2(g) CH4(g)+ H2O(g)
A) 2C(s)+ 2H2O(g) CO2(g)+ CH4(g)
B) 2C(s)+ 3H2O(g) CO(g)+ CO2(g)+ 3H2(g)
C) 2C(s)+ H2O(g)+ H2(g) CO(g)+ CH4(g)
D) 2CO(g)+ 2H2(g) CH4(g)+ CO2(g)
E) 2C(s)+ CH4(g)+ 3H2O(g) CO(g)+ 5H2(g)
2C(s)+ 2H2O(g) 2CO(g)+ 2H2(g)
CO(g)+ H2O(g) CO2(g)+ H2(g)
CO(g)+ 3H2(g) CH4(g)+ H2O(g)
A) 2C(s)+ 2H2O(g) CO2(g)+ CH4(g)
B) 2C(s)+ 3H2O(g) CO(g)+ CO2(g)+ 3H2(g)
C) 2C(s)+ H2O(g)+ H2(g) CO(g)+ CH4(g)
D) 2CO(g)+ 2H2(g) CH4(g)+ CO2(g)
E) 2C(s)+ CH4(g)+ 3H2O(g) CO(g)+ 5H2(g)

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
43
Commercial cold packs consist of solid ammonium nitrate and water.NH4NO3 absorbs 25.69 kJ of heat per mole dissolved in water.In a coffee-cup calorimeter,5.60 g NH4NO3 is dissolved in 100.0 g of water at 22.0 C.What is the final temperature of the solution? Assume that the solution has a specific heat capacity of 4.18 J/g.K.
A) 0.0 C
B) 17.9 C
C) 11.6 C
D) -54.8 C
E) 26.1 C
A) 0.0 C
B) 17.9 C
C) 11.6 C
D) -54.8 C
E) 26.1 C

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
44
Determine rH for the following reaction,
2 NH3(g)+ 5/2 O2(g) 2 NO(g)+ 3 H2O(g)
Given the thermochemical equations below.
A) -1178.2 kJ/mol-rxn
B) -452.8 kJ/mol-rxn
C) -394.6 kJ/mol-rxn
D) -211.0 kJ/mol-rxn
E) +1178.2 kJ/mol-rxn
2 NH3(g)+ 5/2 O2(g) 2 NO(g)+ 3 H2O(g)
Given the thermochemical equations below.

A) -1178.2 kJ/mol-rxn
B) -452.8 kJ/mol-rxn
C) -394.6 kJ/mol-rxn
D) -211.0 kJ/mol-rxn
E) +1178.2 kJ/mol-rxn

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
45
A bomb calorimeter has a heat capacity of 2.47 kJ/K.When a 0.105-g sample of a certain hydrocarbon was burned in this calorimeter,the temperature increased by 2.14 K.Calculate the energy of combustion for 1 g of the hydrocarbon.
A) -5.29 J/g
B)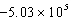 J/g
J/g
C) -0.120 J/g
D)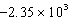 J/g
J/g
E) -0.560 J/g
A) -5.29 J/g
B)
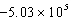 J/g
J/gC) -0.120 J/g
D)
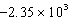 J/g
J/gE) -0.560 J/g

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
46
Determine the standard enthalpy of formation of Fe2O3(s)given the thermochemical equations below.
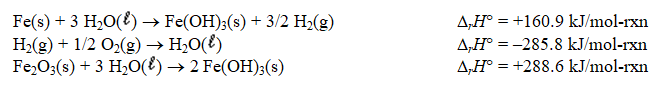
A) -252.6 kJ/mol-rxn
B) +163.7 kJ/mol-rxn
C) -824.2 kJ/mol-rxn
D) +33.2 kJ/mol-rxn
E) + 890.6 kJ/mol-rxn
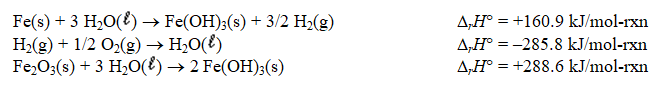
A) -252.6 kJ/mol-rxn
B) +163.7 kJ/mol-rxn
C) -824.2 kJ/mol-rxn
D) +33.2 kJ/mol-rxn
E) + 890.6 kJ/mol-rxn

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
47
Iron oxide reacts with aluminum in an exothermic reaction.
Fe2O3(s)+ 2 Al(s) 2 Fe(s)+ Al2O3(s)
The reaction of 5.00 g Fe2O3 with excess Al(s)evolves 26.6 kJ of energy in the form of heat.Calculate the enthalpy change per mole of Fe2O3 reacted.
A) -5.32 kJ/mol
B) -1.33 102 kJ/mol
C) -2.12 104 kJ/mol
D) -2.12 102 kJ/mol
E) -8.50 102 kJ/mol
Fe2O3(s)+ 2 Al(s) 2 Fe(s)+ Al2O3(s)
The reaction of 5.00 g Fe2O3 with excess Al(s)evolves 26.6 kJ of energy in the form of heat.Calculate the enthalpy change per mole of Fe2O3 reacted.
A) -5.32 kJ/mol
B) -1.33 102 kJ/mol
C) -2.12 104 kJ/mol
D) -2.12 102 kJ/mol
E) -8.50 102 kJ/mol

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
48
How much heat is liberated at constant pressure if 0.834 g of calcium carbonate reacts with 48.9 mL of 0.668 M hydrochloric acid?
CaCO3(s)+ 2HCl(aq) CaCl2(aq)+ H2O(l)+ CO2(g); rH° = -15.2 kJ/mol-rxn
A) -0.127 kJ
B) -0.375 kJ
C) -12.7 kJ
D) -0.248 kJ
E) -10.2 kJ
CaCO3(s)+ 2HCl(aq) CaCl2(aq)+ H2O(l)+ CO2(g); rH° = -15.2 kJ/mol-rxn
A) -0.127 kJ
B) -0.375 kJ
C) -12.7 kJ
D) -0.248 kJ
E) -10.2 kJ

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
49
Hydrazine,N2H4,is a liquid used as a rocket fuel.It reacts with oxygen to yield nitrogen gas and water.
N2H4(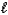 )+ O2(g) N2(g)+ 2 H2O(
)+ O2(g) N2(g)+ 2 H2O( 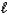 )
)
The reaction of 6.50 g N2H4 evolves 126.2 kJ of heat.Calculate the enthalpy change per mole of hydrazine combusted.
A) -19.4 kJ/mol
B) -25.6 kJ/mol
C) -126 kJ/mol
D) -622 kJ/mol
E) -820.kJ/mol
N2H4(
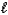 )+ O2(g) N2(g)+ 2 H2O(
)+ O2(g) N2(g)+ 2 H2O( 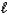 )
)The reaction of 6.50 g N2H4 evolves 126.2 kJ of heat.Calculate the enthalpy change per mole of hydrazine combusted.
A) -19.4 kJ/mol
B) -25.6 kJ/mol
C) -126 kJ/mol
D) -622 kJ/mol
E) -820.kJ/mol

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
50
Combustion of 7.21 g of liquid benzene (C6H6)causes a temperature rise of 50.3°C in a constant-pressure calorimeter that has a heat capacity of 5.99 kJ/°C.What is H for the following reaction?
C6H6(l)+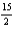
O2(g) 6CO2(g)+ 3H2O(l)
A) -302 kJ/mol-rxn
B) 41.8 kJ/mol-rxn
C) -41.8 kJ/mol-rxn
D) -3.27 103 kJ/mol-rxn
E) 302 kJ/mol-rxn
C6H6(l)+
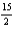
O2(g) 6CO2(g)+ 3H2O(l)
A) -302 kJ/mol-rxn
B) 41.8 kJ/mol-rxn
C) -41.8 kJ/mol-rxn
D) -3.27 103 kJ/mol-rxn
E) 302 kJ/mol-rxn

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
51
Determine the enthalpy change for the decomposition of calcium carbonate
CaCO3(s) CaO(s)+ CO2(g)
Given the thermochemical equations below.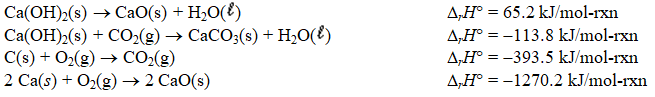
A) +48.6 kJ/mol-rxn
B) +179.0 kJ/mol-rxn
C) +345.5 kJ/mol-rxn
D) +441.0 kJ/mol-rxn
E) +1711.7 kJ/mol-rxn
CaCO3(s) CaO(s)+ CO2(g)
Given the thermochemical equations below.
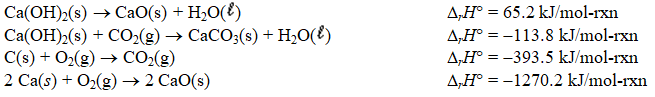
A) +48.6 kJ/mol-rxn
B) +179.0 kJ/mol-rxn
C) +345.5 kJ/mol-rxn
D) +441.0 kJ/mol-rxn
E) +1711.7 kJ/mol-rxn

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
52
When 10.0 g KOH is dissolved in 100.0 g of water in a coffee-cup calorimeter,the temperature rises from 25.18 C to 47.53 C.What is the enthalpy change per gram of KOH dissolved in the water? Assume that the solution has a specific heat capacity of 4.18 J/g.K.
A) -116 J/g
B) -934 J/g
C) -1.03 103 J/g
D) -2.19 103 J/g
E) -1.03 104 J/g
A) -116 J/g
B) -934 J/g
C) -1.03 103 J/g
D) -2.19 103 J/g
E) -1.03 104 J/g

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
53
A chemical reaction in a bomb calorimeter evolves 3.86 kJ of energy in the form of heat.If the temperature of the bomb calorimeter increases by 4.17 K,what is the heat capacity of the calorimeter?
A) 3.87 103 J/K
B) 311 J/K
C) 926 J/K
D) 1.8 103 J/K
E) 1.61 104 J/K
A) 3.87 103 J/K
B) 311 J/K
C) 926 J/K
D) 1.8 103 J/K
E) 1.61 104 J/K

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
54
When 50.0 mL of 1.30 M of HCl(aq)is combined with 50.0 mL of 1.20 M of NaOH(aq)in a coffee-cup calorimeter,the temperature of the solution increases by 8.01°C.What is the change in enthalpy for this balanced reaction?
HCl(aq)+ NaOH(aq) NaCl(aq)+ H2O(l)
Assume that the solution density is 1.00 g/mL and the specific heat capacity of the solution is 4.18 J/g. C.
A) -55.8 kJ
B) 55.8 kJ
C) 51.5 kJ
D) -51.5 kJ
E) -26.8 kJ
HCl(aq)+ NaOH(aq) NaCl(aq)+ H2O(l)
Assume that the solution density is 1.00 g/mL and the specific heat capacity of the solution is 4.18 J/g. C.
A) -55.8 kJ
B) 55.8 kJ
C) 51.5 kJ
D) -51.5 kJ
E) -26.8 kJ

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
55
Using the following thermochemical data:
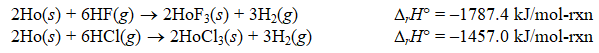
Calculate rH for the following reaction:
HoF3(s)+ 3HCl(g) HoCl3(s)+ 3HF(g)
A) -3244.4 kJ/mol-rxn
B) 330.4 kJ/mol-rxn
C) 165.2 kJ/mol-rxn
D) 660.8 kJ/mol-rxn
E) -1622.2 kJ/mol-rxn
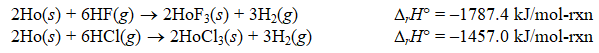
Calculate rH for the following reaction:
HoF3(s)+ 3HCl(g) HoCl3(s)+ 3HF(g)
A) -3244.4 kJ/mol-rxn
B) 330.4 kJ/mol-rxn
C) 165.2 kJ/mol-rxn
D) 660.8 kJ/mol-rxn
E) -1622.2 kJ/mol-rxn

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
56
CaO(s)reacts with water to form Ca(OH)2(aq).If 6.50 g CaO is combined with 99.70 g H2O in a coffee cup calorimeter,the temperature of the resulting solution increases from 21.7 C to 43.1 C.Calculate the enthalpy change for the reaction per mole of CaO.Assume that the specific heat capacity of the solution is 4.18 J/g.K.
A) -1.45 kJ/mol
B) -82.0 kJ/mol
C) -9.42 kJ/mol
D) -165 kJ/mol
E) -532 kJ/mol
A) -1.45 kJ/mol
B) -82.0 kJ/mol
C) -9.42 kJ/mol
D) -165 kJ/mol
E) -532 kJ/mol

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
57
A bomb calorimeter has a heat capacity of 2.47 kJ/K.When a 0.123-g sample of ethylene (C2H4)was burned in this calorimeter,the temperature increased by 2.50 K.Calculate the enthalpy change per mole of ethylene combusted.
A) -5.29 kJ/mol
B) -50.2 kJ/mol
C) -563 kJ/mol
D) -0.304 kJ/mol
E) -1.41 103 kJ/mol
A) -5.29 kJ/mol
B) -50.2 kJ/mol
C) -563 kJ/mol
D) -0.304 kJ/mol
E) -1.41 103 kJ/mol

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
58
When 0.236 mol of a weak base (A-)is reacted with excess HCl,6.91 kJ of energy is released as heat.What is H for this reaction per mole of A- consumed?
A) -34.2 kJ/mol
B) -59.4 kJ/mol
C) -29.3 kJ/mol
D) 34.2 kJ/mol
E) 29.3 kJ/mol
A) -34.2 kJ/mol
B) -59.4 kJ/mol
C) -29.3 kJ/mol
D) 34.2 kJ/mol
E) 29.3 kJ/mol

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
59
Acetylene,C2H2,is a gas used in welding.The molar enthalpy of combustion for acetylene is -2599 kJ.A mass of 0.338 g C2H2(g)is combusted in a bomb calorimeter.If the heat capacity of the calorimeter is 729 J/K and it contains 1.150 kg of water,what is the temperature increase of the bomb calorimeter? The specific heat capacity of water is 4.184 J/g.K and the molar mass of acetylene is 26.04 g/mol.
A) 1.59 K
B) 6.09 K
C) 7.01 K
D) 12.3 K
E) 18.0 K
A) 1.59 K
B) 6.09 K
C) 7.01 K
D) 12.3 K
E) 18.0 K

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
60
Determine the heat of evaporation of carbon disulfide,
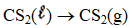
Given the enthalpies of reaction below.

A) -206.1 kJ
B) -27.3 kJ
C) +27.3 kJ
D) +206.1 kJ
E) +1.31 kJ
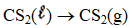
Given the enthalpies of reaction below.

A) -206.1 kJ
B) -27.3 kJ
C) +27.3 kJ
D) +206.1 kJ
E) +1.31 kJ

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
61
The standard molar enthalpy of formation of NH3(g)is -45.9 kJ/mol.What is the enthalpy change if 9.51 g N2(g)and 1.96 g H2(g)react to produce NH3(g)?
A) -10.3 kJ/mol-rxn
B) -20.7 kJ/mol-rxn
C) -29.8 kJ/mol-rxn
D) -43.7 kJ/mol-rxn
E) -65.6 kJ/mol-rxn
A) -10.3 kJ/mol-rxn
B) -20.7 kJ/mol-rxn
C) -29.8 kJ/mol-rxn
D) -43.7 kJ/mol-rxn
E) -65.6 kJ/mol-rxn

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
62
Dry ice converts directly from a solid to a gas when heated.This process is called ________.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
63
When 1 mole of Fe2O3(s)reacts with H2(g)to form Fe(s)and H2O(g)according to the following equation,98.8 kJ of energy are absorbed.
Fe2O3(s)+ 3 H2(g) 2 Fe(s)+ 3 H2O(g)
(A)
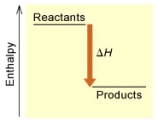
(B)
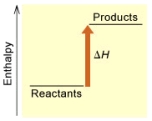
Is the reaction endothermic or exothermic,and which of the enthalpy diagrams above
Represents this reaction?
A) endothermic,A
B) endothermic,B
C) exothermic,A
D) exothermic,B
Fe2O3(s)+ 3 H2(g) 2 Fe(s)+ 3 H2O(g)
(A)

(B)

Is the reaction endothermic or exothermic,and which of the enthalpy diagrams above
Represents this reaction?
A) endothermic,A
B) endothermic,B
C) exothermic,A
D) exothermic,B

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
64
The standard enthalpy change for the combustion of 1 mole of propane is -2043.0 kJ.C3H8(g)+ 5 O2(g) 3 CO2(g)+ 4 H2O(g)
Calculate fH for propane based on the following standard molar enthalpies of formation.
A) -1407.7 kJ/mol-rxn
B) +104.7 kJ/mol-rxn
C) -104.7 kJ/mol-rxn
D) -4190.7 kJ/mol-rxn
E) +1407.7 kJ/mol-rxn
Calculate fH for propane based on the following standard molar enthalpies of formation.

A) -1407.7 kJ/mol-rxn
B) +104.7 kJ/mol-rxn
C) -104.7 kJ/mol-rxn
D) -4190.7 kJ/mol-rxn
E) +1407.7 kJ/mol-rxn

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
65
The heat required to convert a solid at its melting point to a liquid is called the heat of ________.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
66
Calculate rH for the combustion of ammonia,
4 NH3(g)+ 7 O2(g) 4 NO2(g)+ 6 H2O(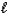 )
)
Using standard molar enthalpies of formation.

A) +30.24 kJ/mol-rxn
B) -206.9 kJ/mol-rxn
C) -298.6 kJ/mol-rxn
D) -1398.8 kJ/mol-rxn
E) -1663.6 kJ/mol-rxn
4 NH3(g)+ 7 O2(g) 4 NO2(g)+ 6 H2O(
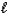 )
)Using standard molar enthalpies of formation.

A) +30.24 kJ/mol-rxn
B) -206.9 kJ/mol-rxn
C) -298.6 kJ/mol-rxn
D) -1398.8 kJ/mol-rxn
E) -1663.6 kJ/mol-rxn

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
67
Internal energy and enthalpy are state functions.What is meant by this statement?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
68
In thermodynamics,a(n)________ is defined as the object,or collection of objects,being studied.The surroundings include everything else.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following chemical equations does not correspond to a standard molar enthalpy of formation?
A) Mg(s)+ C(s)+ 3/2 O2(g) MgCO3(s)
B) C(s)+ 1/2 O2(g) CO(g)
C) N2(g)+ O2(g) 2 NO(g)
D) N2(g)+ 2 O2(g) N2O4(g)
E) H2(g)+ 1/2 O2(g) H2O(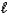 )
)
A) Mg(s)+ C(s)+ 3/2 O2(g) MgCO3(s)
B) C(s)+ 1/2 O2(g) CO(g)
C) N2(g)+ O2(g) 2 NO(g)
D) N2(g)+ 2 O2(g) N2O4(g)
E) H2(g)+ 1/2 O2(g) H2O(
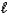 )
)
Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following reactions corresponds to the thermochemical equation for the standard molar enthalpy of formation of solid calcium nitrate?
A) Ca2+(aq)+ 2NO3-(aq) Ca(NO3)2(s)
B) Ca(OH)2(s)+ 2HNO3(aq) Ca(NO3)2(s)+ 2H2O(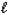 )
)
C) Ca(s)+ N2(g)+ 3O2(g) Ca(NO3)2(s)
D) Ca(s)+ 2HNO3(aq) Ca(NO3)2(s)+ H2(g)
E) Ca(s)+ 2N(g)+ 6O(g) Ca(NO3)2(s)
A) Ca2+(aq)+ 2NO3-(aq) Ca(NO3)2(s)
B) Ca(OH)2(s)+ 2HNO3(aq) Ca(NO3)2(s)+ 2H2O(
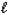 )
)C) Ca(s)+ N2(g)+ 3O2(g) Ca(NO3)2(s)
D) Ca(s)+ 2HNO3(aq) Ca(NO3)2(s)+ H2(g)
E) Ca(s)+ 2N(g)+ 6O(g) Ca(NO3)2(s)

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
71
What is rH° for the following phase change?
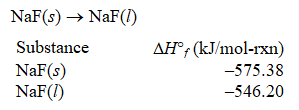
A) 1121.58 kJ/mol-rxn
B) 29.18 kJ/mol-rxn
C) -1121.58 kJ/mol-rxn
D) -29.18 kJ/mol-rxn
E) 0 kJ/mol-rxn
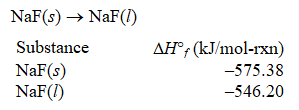
A) 1121.58 kJ/mol-rxn
B) 29.18 kJ/mol-rxn
C) -1121.58 kJ/mol-rxn
D) -29.18 kJ/mol-rxn
E) 0 kJ/mol-rxn

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
72
Why are you at greater risk from being burned by steam at 100 C than from liquid water at the same temperature?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
73
What is the standard enthalpy of formation of BaCO3(s)?
BaO(s)+ CO2(g) BaCO3(s); H° = -269.3 kJ/mol-rxn
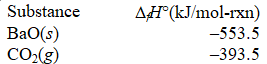
A) -109.3 kJ/mol-rxn
B) -429.3 kJ/mol-rxn
C) -677.7 kJ/mol-rxn
D) -1216.3 kJ/mol-rxn
E) 677.7 kJ/mol-rxn
BaO(s)+ CO2(g) BaCO3(s); H° = -269.3 kJ/mol-rxn
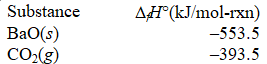
A) -109.3 kJ/mol-rxn
B) -429.3 kJ/mol-rxn
C) -677.7 kJ/mol-rxn
D) -1216.3 kJ/mol-rxn
E) 677.7 kJ/mol-rxn

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
74
Determine the standard enthalpy of formation of calcium carbonate from the thermochemical equations given below.
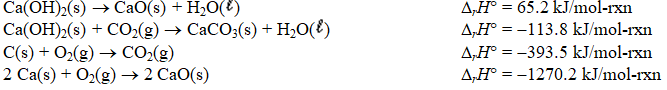
A) (-1712.3 kJ/mol-rxn)
B) (-441.8 kJ/mol-rxn)
C) (-849.6 kJ/mol-rxn)
D) (-980.6 kJ/mol-rxn)
E) (-1207.6 kJ/mol-rxn)
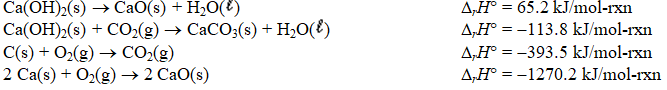
A) (-1712.3 kJ/mol-rxn)
B) (-441.8 kJ/mol-rxn)
C) (-849.6 kJ/mol-rxn)
D) (-980.6 kJ/mol-rxn)
E) (-1207.6 kJ/mol-rxn)

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck


