Deck 17: Structure Determination 1: Ultravioletvisible and Infrared Spectroscopies
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/50
Play
Full screen (f)
Deck 17: Structure Determination 1: Ultravioletvisible and Infrared Spectroscopies
1
Which of the following would you expect to exhibit the strongest C 
C stretching absorption band in its IR spectrum?
A)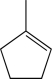
B)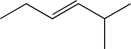
C)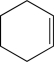
D)
E)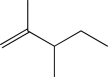

C stretching absorption band in its IR spectrum?
A)

B)

C)

D)

E)


2
Which of the electron transitions shown in the figure below would correspond to the UV-vis absorption band at the longest wavelength? 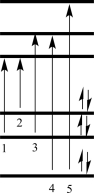
A)1
B)2
C)3
D)4
E)5

A)1
B)2
C)3
D)4
E)5
2
3
Which of the following would show a C 
O stretch at the lowest frequency in its IR spectrum?
A)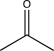
B)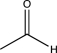
C)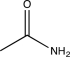
D)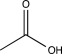
E)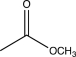

O stretch at the lowest frequency in its IR spectrum?
A)

B)

C)

D)

E)


4
Which of the following would be likely to exhibit an absorption band of moderate to strong intensity at 2240 cm1?
A)
B)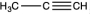
C)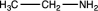
D)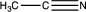
E)
A)

B)
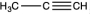
C)
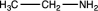
D)
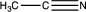
E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following corresponds to the greatest proportion of light absorbed by a sample?
A)50%T
B)75%T
C)25%T
D)100%T
E)15%T
A)50%T
B)75%T
C)25%T
D)100%T
E)15%T

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following bonds would have the lowest stretching vibrational frequency?
A)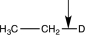
B)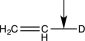
C)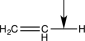
D)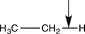
E)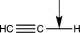
A)

B)

C)

D)

E)
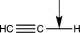

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following structural features is consistent with the spectrum shown below? 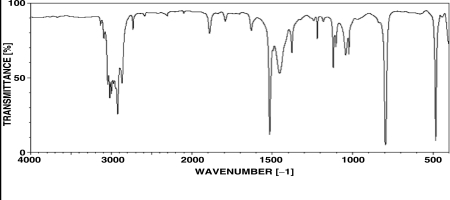
A)An unsubstituted benzene ring
B)A monosubstituted benzene ring
C)An ortho-substituted benzene ring
D)A meta-substituted benzene ring
E)A para-substituted benzene ring

A)An unsubstituted benzene ring
B)A monosubstituted benzene ring
C)An ortho-substituted benzene ring
D)A meta-substituted benzene ring
E)A para-substituted benzene ring

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
8
What is the approximate energy difference between the HOMO and LUMO of a molecule that shows absorption bands at 286 nm,438 nm,and 472 nm in its UV-vis spectrum?
A)4.54 × 10-19 J
B)6.95 × 10-19 J
C)7.24 × 10-19 J
D)3.98 × 10-19 J
E)4.21 × 10-19 J
A)4.54 × 10-19 J
B)6.95 × 10-19 J
C)7.24 × 10-19 J
D)3.98 × 10-19 J
E)4.21 × 10-19 J

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements is false?
A)A photon's energy increases as its frequency increases.
B)As a photon's wavelength increases, its frequency increases.
C)As the energy of a photon decreases, its wavelength increases.
D)The wavelength of a photon increases as its frequency decreases.
E)A photon with the longest wavelength has the lowest energy.
A)A photon's energy increases as its frequency increases.
B)As a photon's wavelength increases, its frequency increases.
C)As the energy of a photon decreases, its wavelength increases.
D)The wavelength of a photon increases as its frequency decreases.
E)A photon with the longest wavelength has the lowest energy.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
10
If we wished to monitor a frequency to determine whether the reaction below had taken place,which frequency would be the best choice? 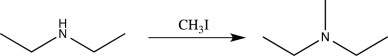
A)2200 cm-1
B)1600 cm-1
C)3300 cm-1
D)1050 cm-1
E)2950 cm-1

A)2200 cm-1
B)1600 cm-1
C)3300 cm-1
D)1050 cm-1
E)2950 cm-1

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following would not show any peaks at frequencies higher than 3000 cm-1 in its IR spectrum?
A)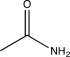
B)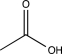
C)
D)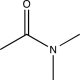
E)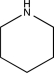
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
12
A UV-vis radiation source has an intensity of 1.0 × 10-3 W.The radiation is sent through an analyte,and the detector measures an intensity of 2.8 × 10-4 W.What is the absorbance of the sample?
A)0.28
B)3.6
C)0.72
D)0.55
E)1.0
A)0.28
B)3.6
C)0.72
D)0.55
E)1.0

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following stretching vibrations will appear at the highest frequency?
A)
B)
C)
D)
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following would be expected to have the highest-intensity peak near 2950 cm-1?
A)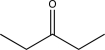
B)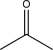
C)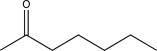
D)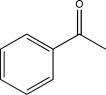
E)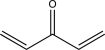
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following wavelengths corresponds to the photon with the highest energy?
A)385 nm
B)425 nm
C)318 nm
D)538 nm
E)647 nm
A)385 nm
B)425 nm
C)318 nm
D)538 nm
E)647 nm

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following would you expect to have the longest λmax?
A)
B)
C)
D)
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
17
What type of electron transition would create an absorption band at the longest wavelength in the UV-vis spectrum of the molecule below? 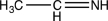
A)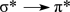
B)
C)
D)
E)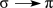
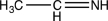
A)
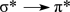
B)

C)

D)

E)
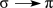

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following choices correctly ranks the molecules shown below in order of increasing stretching vibrational frequency? 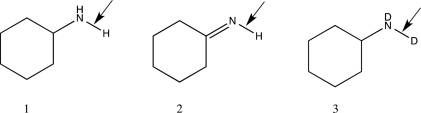
A)1 < 2 < 3
B)1 < 3 < 2
C)3 < 1 < 2
D)3 < 2 < 1
E)2 < 1 < 3

A)1 < 2 < 3
B)1 < 3 < 2
C)3 < 1 < 2
D)3 < 2 < 1
E)2 < 1 < 3

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
19
Identify the following vibrational mode. 
A)Symmetric stretch
B)Asymmetric stretch
C)Out-of-phase bend
D)In-phase bend
E)More than one of the above

A)Symmetric stretch
B)Asymmetric stretch
C)Out-of-phase bend
D)In-phase bend
E)More than one of the above

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
20
When the concentration of an analyte increases by a factor of three,what will happen to the UV-vis absorbance?
A)It will decrease by a factor of three.
B)It will increase by a factor of one-third.
C)It will increase by a factor of three.
D)It will decrease by a factor one-third.
E)It will increase by a factor of log(3).
A)It will decrease by a factor of three.
B)It will increase by a factor of one-third.
C)It will increase by a factor of three.
D)It will decrease by a factor one-third.
E)It will increase by a factor of log(3).

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
21
Rank the following bonds in order of increasing stretching vibration frequency. 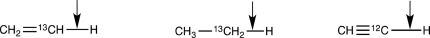

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
22
Label the axes on the UV-vis spectrum below. 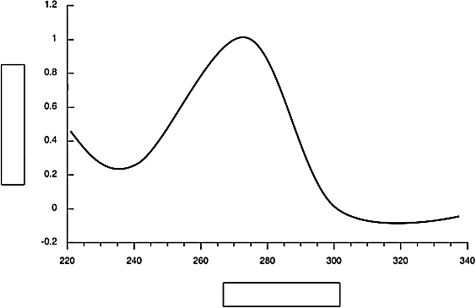


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
23
Indicate which of the following bonds would have the higher stretching vibrational frequency.Explain your answer. 


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
24
Draw a structural isomer of the molecule shown below that would have a shorter λmax. 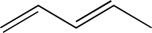


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
25
Draw the symmetric N-H stretch that would occur in the molecule below. 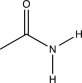


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the two isomers shown below would exhibit a stronger C 
C stretching absorption band? Explain your answer.

C stretching absorption band? Explain your answer.


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following bonds would have a higher stretching frequency? Explain. 


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following structural features is most consistent with the spectrum shown below? 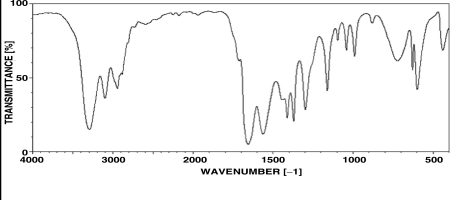
A)Secondary amide
B)Ketone
C)Primary amine
D)Aldehyde
E)Secondary alcohol

A)Secondary amide
B)Ketone
C)Primary amine
D)Aldehyde
E)Secondary alcohol

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
29
Which structure is most consistent with the IR spectrum shown below? 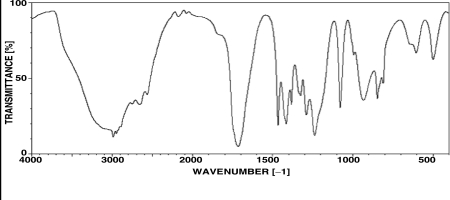
A)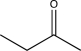
B)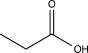
C)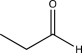
D)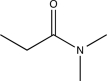
E)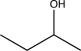

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following would you expect to have the longer λmax? Explain. 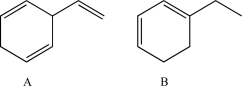


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
31
Label the axes on the IR spectrum below. 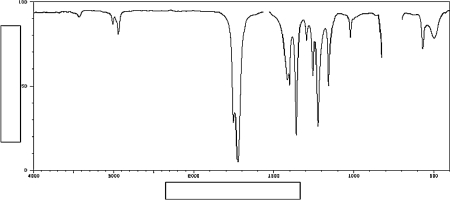


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
32
Rank the following in order of increasing C 
O stretching frequency.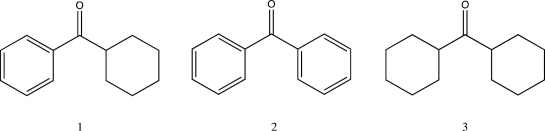
A)2 < 1 < 3
B)3 < 1 < 2
C)1 < 2 < 3
D)2 < 3 < 1
E)3 < 2 < 1

O stretching frequency.

A)2 < 1 < 3
B)3 < 1 < 2
C)1 < 2 < 3
D)2 < 3 < 1
E)3 < 2 < 1

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
33
An unknown compound with the molecular formula C4H7N produces the IR spectrum shown below.What is the most likely identity of the unknown compound? 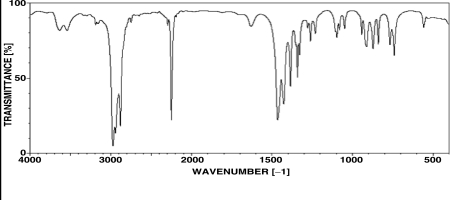
A)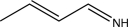
B)
C)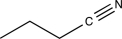
D)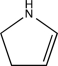
E)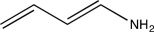

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following compounds is consistent with the IR spectrum shown below? 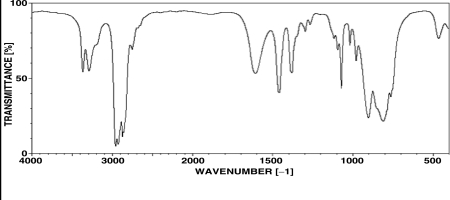
A)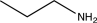
B)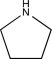
C)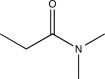
D)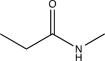
E)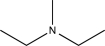

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
35
How could you distinguish between the triple-bond stretches in the IR spectra of the two molecules shown below? 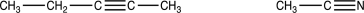
A B
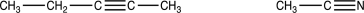
A B

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
36
A sample has an absorbance of 0.68 at a particular wavelength in the UV-vis region.The intensity of the radiation source in the spectrophotometer was 1.5 × 10-4 W.What was the intensity of radiation measured by the detector?

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
37
Calculate the approximate wavelength (in nm)required to excite the vibrational frequency indicated in the spectrum below. 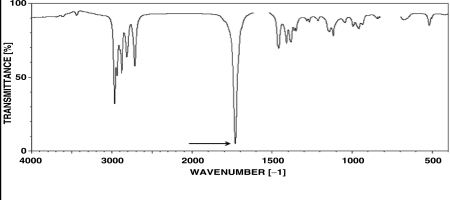


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
38
Based on the spectrum below,calculate the approximate energy difference (in J)between the HOMO and LUMO of the molecule that created the spectrum. 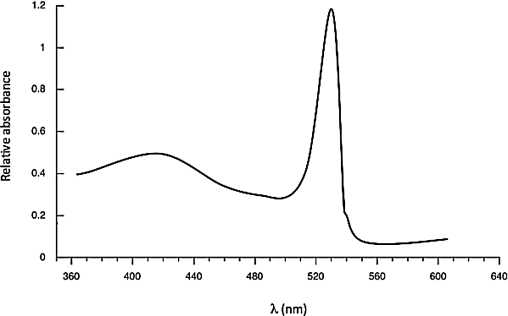


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
39
Briefly explain why acetone absorbs at a longer λmax than isobutylene. 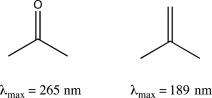


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the two molecules below would you expect to have a longer λmax? 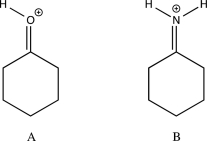


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
41
An unknown compound with a molecular formula C8H8O2 produced the IR spectrum shown here. Draw a structure that is consistent with the spectrum. 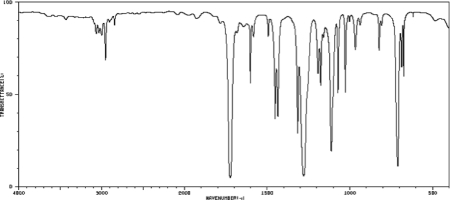


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
42
A compound whose molecular formula is C5H8O shows a strong absorption band at 1692 cm1.Draw a structure consistent with this information.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
43
A compound has the molecular formula C7H6N2.Determine its structure based on the spectrum below. 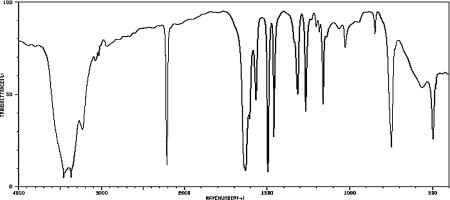


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
44
Describe how you could use IR spectroscopy to distinguish between the two isomers shown below. 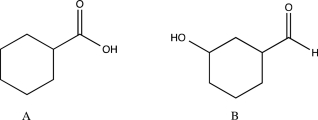


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
45
What carbonyl-containing functional group is indicated by the IR spectrum below? 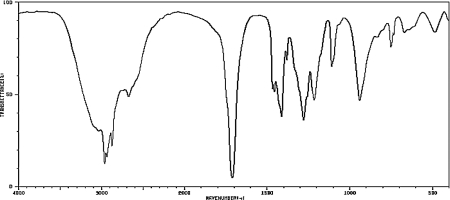


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following would you expect to have the lower-frequency C 
O stretch? Explain.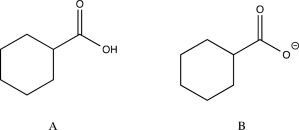

O stretch? Explain.


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
47
What two functional groups are likely indicated by the peaks labeled A and B in the IR spectrum below? 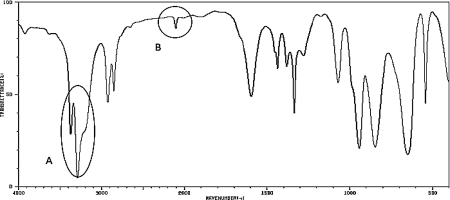


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
48
Match each compound below to its IR spectrum based on the intensity of the alkane CH stretch absorption band. 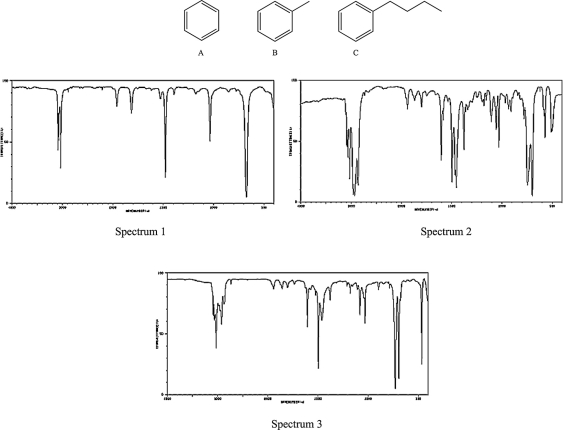


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
49
An unknown compound has the molecular formula C3H7ON.Draw a structure of the unknown that is consistent with the IR spectrum below. 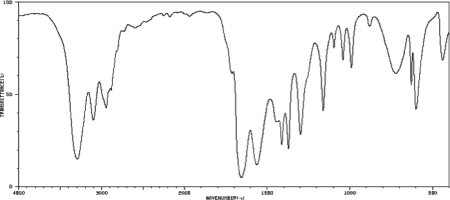


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following compounds would be consistent with the IR spectrum shown below? Briefly explain your reasoning. 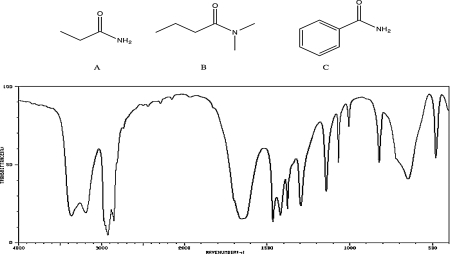


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck


