Exam 17: Structure Determination 1: Ultravioletvisible and Infrared Spectroscopies
Exam 1: Atomic and Molecular Structure54 Questions
Exam 2: Interchapter: Nomenclature 1-477 Questions
Exam 3: Interchapter 1molecular Orbital Theory and Chemical Reactions17 Questions
Exam 4: Three-Dimensional Geometry,intermolecular Interactions,and Physical Properties53 Questions
Exam 5: Orbital Interactions 1: Hybridization and Two-Center Molecular Orbitals56 Questions
Exam 6: Isomerism 1: Conformational and Constitutional Isomers60 Questions
Exam 7: Isomerism 2: Chirality,enantiomers,and Diastereomers63 Questions
Exam 8: The Proton Transfer Reaction: an Introduction to Mechanisms,thermodynamics,and Charge Stability51 Questions
Exam 9: An Overview of the Most Common Elementary Steps58 Questions
Exam 10: An Introduction to Multistep Mechanisms: Sn1 and E1 Reactions59 Questions
Exam 11: Nucleophilic Substitution and Elimination Reactions 1: Competition Among Sn2,sn1,e2,and E1 Reactions50 Questions
Exam 12: Nucleophilic Substitution and Elimination Reactions 2: Reactions That Are Useful for Synthesis58 Questions
Exam 13: Electrophilic Addition to Nonpolar Π Bonds 1: Addition of a Brønsted Acid50 Questions
Exam 14: Electrophilic Addition to Nonpolar Π Bonds 2: Reactions Involving Cyclic Transition States55 Questions
Exam 15: Organic Synthesis 1: Beginning Concepts50 Questions
Exam 16: Orbital Interactions 2: Extended Π Systems,conjugation,and Aromaticity54 Questions
Exam 17: Structure Determination 1: Ultravioletvisible and Infrared Spectroscopies50 Questions
Exam 18: Structure Determination 2: Nuclear Magnetic Resonance Spectroscopy and Mass Spectrometry60 Questions
Exam 19: Nucleophilic Addition to Polar Π Bonds 1: Addition of Strong Nucleophiles50 Questions
Exam 20: Nucleophilic Addition to Polar Π Bonds 2: Addition of Weak Nucleophiles and Acid and Base Catalysis63 Questions
Exam 21: Organic Synthesis 2: Intermediate Topics in Synthesis Design,and Useful Reduction and Oxidation Reactions50 Questions
Exam 22: Nucleophilic Additionelimination Reactions 1: the General Mechanism Involving Strong Nucleophiles55 Questions
Exam 23: Nucleophilic Additionelimination Reactions 2: Weak Nucleophiles54 Questions
Exam 24: Electrophilic Aromatic Substitution 1: Substitution on Benzene; Useful Accompanying Reactions50 Questions
Exam 25: Electrophilic Aromatic Substitution 2: Substitution on Mono- and Disubstituted Benzene and Other Aromatic Rings50 Questions
Exam 26: The Dielsalder Reaction and Other Pericyclic Reactions53 Questions
Exam 27: Reactions Involving Free Radicals50 Questions
Exam 28: Polymers51 Questions
Select questions type
When the concentration of an analyte increases by a factor of three,what will happen to the UV-vis absorbance?
Free
(Multiple Choice)
4.8/5  (29)
(29)
Correct Answer:
C
Which of the following structural features is consistent with the spectrum shown below? 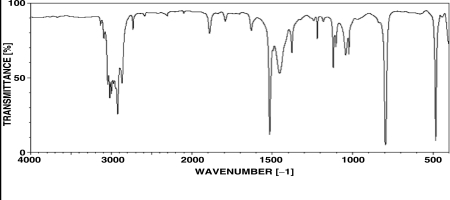
Free
(Multiple Choice)
4.7/5  (25)
(25)
Correct Answer:
E
Which of the following would you expect to have the lower-frequency C  O stretch? Explain.
O stretch? Explain. 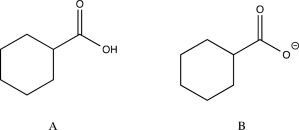
Free
(Essay)
4.8/5  (46)
(46)
Correct Answer:
The carboxylate anion (B)would show a lower-frequency C  O stretch than the carboxylic acid (A).This is because it contains a significant resonance contributor in which the negative charge is localized on the other oxygen,as shown below.Therefore,the double bond in B is weaker (in fact,it is more accurately a bond with an order of 1.5 rather than a actual double bond),and a weaker bond corresponds to a lower frequency.
O stretch than the carboxylic acid (A).This is because it contains a significant resonance contributor in which the negative charge is localized on the other oxygen,as shown below.Therefore,the double bond in B is weaker (in fact,it is more accurately a bond with an order of 1.5 rather than a actual double bond),and a weaker bond corresponds to a lower frequency. 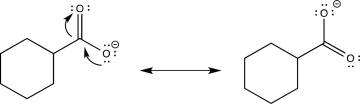
Which structure is most consistent with the IR spectrum shown below? 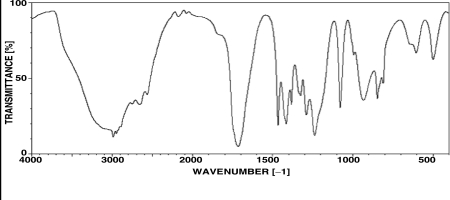
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following would you expect to have the longer λmax? Explain. 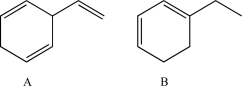
(Essay)
4.8/5  (39)
(39)
Which of the following structural features is most consistent with the spectrum shown below? 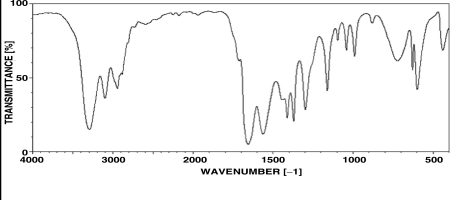
(Multiple Choice)
4.8/5  (34)
(34)
An unknown compound with the molecular formula C4H7N produces the IR spectrum shown below.What is the most likely identity of the unknown compound? 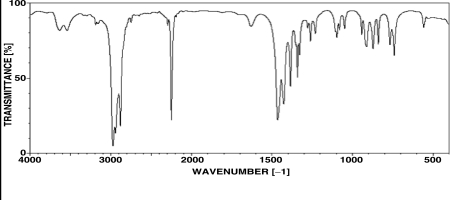
(Multiple Choice)
4.9/5  (42)
(42)
Which of the following would be likely to exhibit an absorption band of moderate to strong intensity at 2240 cm1?
(Multiple Choice)
4.8/5  (35)
(35)
A sample has an absorbance of 0.68 at a particular wavelength in the UV-vis region.The intensity of the radiation source in the spectrophotometer was 1.5 × 10-4 W.What was the intensity of radiation measured by the detector?
(Short Answer)
4.8/5  (38)
(38)
Which of the following would be expected to have the highest-intensity peak near 2950 cm-1?
(Multiple Choice)
4.7/5  (33)
(33)
Which of the electron transitions shown in the figure below would correspond to the UV-vis absorption band at the longest wavelength? 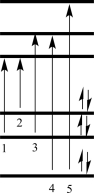
(Multiple Choice)
4.9/5  (33)
(33)
A UV-vis radiation source has an intensity of 1.0 × 10-3 W.The radiation is sent through an analyte,and the detector measures an intensity of 2.8 × 10-4 W.What is the absorbance of the sample?
(Multiple Choice)
4.9/5  (36)
(36)
Draw the symmetric N-H stretch that would occur in the molecule below. 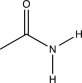
(Short Answer)
4.8/5  (31)
(31)
Calculate the approximate wavelength (in nm)required to excite the vibrational frequency indicated in the spectrum below. 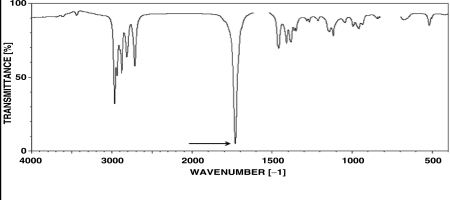
(Short Answer)
4.7/5  (45)
(45)
Match each compound below to its IR spectrum based on the intensity of the alkane CH stretch absorption band. 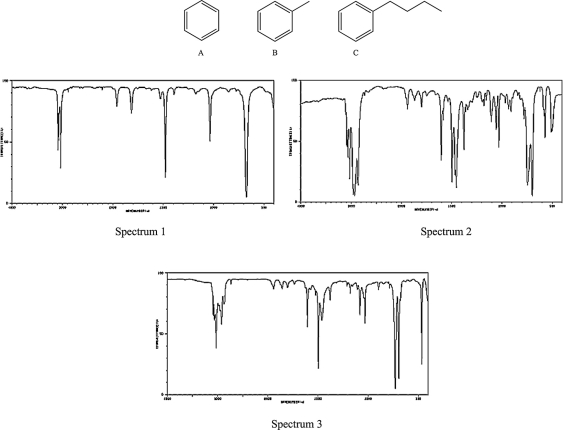
(Essay)
4.8/5  (28)
(28)
Which of the following bonds would have a higher stretching frequency? Explain. 
(Essay)
4.8/5  (43)
(43)
If we wished to monitor a frequency to determine whether the reaction below had taken place,which frequency would be the best choice? 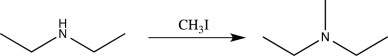
(Multiple Choice)
4.9/5  (44)
(44)
Rank the following bonds in order of increasing stretching vibration frequency. 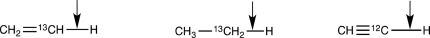
(Short Answer)
4.8/5  (41)
(41)
Which of the following would not show any peaks at frequencies higher than 3000 cm-1 in its IR spectrum?
(Multiple Choice)
4.9/5  (28)
(28)
Showing 1 - 20 of 50
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)