Deck 12: Chemical Logic of Metabolism
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/24
Play
Full screen (f)
Deck 12: Chemical Logic of Metabolism
1
In stage ____ metabolism,a catabolic pathway might produce ________ while the opposing anabolic pathway might produce _________.
A)2;monosaccharides;polysaccharides
B)3;CO2;glucose
C)2;acetyl CoA;lipids
D)1;amino acids;proteins
E)3;NH3;nucleotides
A)2;monosaccharides;polysaccharides
B)3;CO2;glucose
C)2;acetyl CoA;lipids
D)1;amino acids;proteins
E)3;NH3;nucleotides
D
2
Based on the following reaction,what is the respiratory quotient for stearic acid?
C18H36O2 + 26 O2 18 CO2 + 18 H2O
A)1.44
B)0.69
C)1.39
D)0.72
E)none of the above
C18H36O2 + 26 O2 18 CO2 + 18 H2O
A)1.44
B)0.69
C)1.39
D)0.72
E)none of the above
0.69
3
Which of the following pairs of pathways,if active at the same time,would be considered a futile cycle?
A)gluconeogenesis and citric acid cycle
B)fatty acid -oxidation and glycolysis
C)fatty acid synthesis and glycolysis
D)fatty acid synthesis and citric acid cycle
E)glycolysis and gluconeogenesis
A)gluconeogenesis and citric acid cycle
B)fatty acid -oxidation and glycolysis
C)fatty acid synthesis and glycolysis
D)fatty acid synthesis and citric acid cycle
E)glycolysis and gluconeogenesis
glycolysis and gluconeogenesis
4
Which of the following functional groups is a commonly seen nucleophile in biochemical reactions?
A)aldehyde
B)deprotonated alcohol
C)amide
D)carbocation
E)iodide ion
A)aldehyde
B)deprotonated alcohol
C)amide
D)carbocation
E)iodide ion

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following eukaryotic organisms is both autotrophic and heterotrophic?
A)yeast
B)humans
C)Venus fly trap
D)redwood trees
E)mushrooms
A)yeast
B)humans
C)Venus fly trap
D)redwood trees
E)mushrooms

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following functional groups is commonly the electrophile in nucleophilic acyl substitutions?
A)ester
B)hydroxide ion
C)amine
D)thiol
E)carboxylate
A)ester
B)hydroxide ion
C)amine
D)thiol
E)carboxylate

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following would be considered a biosynthetic pathway?
A)glycolysis
B)glycogen synthesis
C)fatty acid -oxidation
D)citric acid cycle
E)electron transport
A)glycolysis
B)glycogen synthesis
C)fatty acid -oxidation
D)citric acid cycle
E)electron transport

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
8
The concentration of which of the following ions can make a significant change in the G for reactions that involve ATP?
A)Mg2+
B)Na+
C)Cl-
D)K+
E)Ca2+
A)Mg2+
B)Na+
C)Cl-
D)K+
E)Ca2+

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements regarding the general concepts of metabolism is true?
A)once glucose is converted to pyruvate,there is no way to convert pyruvate back to glucose
B)when triacylglycerols are used as fuel,only the fatty acids can be metabolized,the glycerol is a waste product
C)amino acids serve as substrates for many pathways including glycolysis and the citric acid cycle
D)the citric acid cycle is generally considered an anabolic pathway while gluconeogenesis is generally considered a catabolic pathway
E)none of the above
A)once glucose is converted to pyruvate,there is no way to convert pyruvate back to glucose
B)when triacylglycerols are used as fuel,only the fatty acids can be metabolized,the glycerol is a waste product
C)amino acids serve as substrates for many pathways including glycolysis and the citric acid cycle
D)the citric acid cycle is generally considered an anabolic pathway while gluconeogenesis is generally considered a catabolic pathway
E)none of the above

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following types of reactions best describes the reaction shown below? 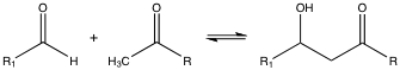
A)nucleophilic substitution
B)nucleophilic addition
C)nucleophilic acyl substitution
D)elimination
E)carbonyl condensation

A)nucleophilic substitution
B)nucleophilic addition
C)nucleophilic acyl substitution
D)elimination
E)carbonyl condensation

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following represents a correct compartmentation of a biochemical process with its cellular location?
A)fatty acid oxidation: endoplasmic reticulum
B)RNA synthesis: Golgi complex
C)citric acid cycle: mitochondria
D)gluconeogenesis: lysosome
E)none of the above
A)fatty acid oxidation: endoplasmic reticulum
B)RNA synthesis: Golgi complex
C)citric acid cycle: mitochondria
D)gluconeogenesis: lysosome
E)none of the above

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
12
Of the following,which would have the most reduced state of carbon,and therefore,would yield the largest amount of energy per gram?
A)glucose
B)glycogen
C)alanine
D)stearic acid
E)all of the above would have identical energy yields
A)glucose
B)glycogen
C)alanine
D)stearic acid
E)all of the above would have identical energy yields

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following accurately describes the adenylate energy charge?
A)numerator: 2 [ATP] + [ADP];denominator: [ATP] + [ADP] + [AMP]
B)numerator: [ATP] + [ADP];denominator: [ATP] + 0.5 [ADP] + 0.25 [AMP]
C)numerator: [ATP] + 0.5 [ADP];denominator: 2 [ATP] + [ADP] + 0.5 [AMP]
D)numerator: [ATP] + 0.5 [ADP];denominator: [ATP] + [ADP] + [AMP]
E)none of the above
A)numerator: 2 [ATP] + [ADP];denominator: [ATP] + [ADP] + [AMP]
B)numerator: [ATP] + [ADP];denominator: [ATP] + 0.5 [ADP] + 0.25 [AMP]
C)numerator: [ATP] + 0.5 [ADP];denominator: 2 [ATP] + [ADP] + 0.5 [AMP]
D)numerator: [ATP] + 0.5 [ADP];denominator: [ATP] + [ADP] + [AMP]
E)none of the above

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
14
The formation of a hemiacetal when glucose undergoes cyclization represents what type of reaction?
A)nucleophilic substitution
B)nucleophilic addition
C)carbonyl condensation
D)elimination
E)oxidation
A)nucleophilic substitution
B)nucleophilic addition
C)carbonyl condensation
D)elimination
E)oxidation

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
15
During the oxidation of glucose within a typical animal cell,reduced electron carrier such as NADH are produced.What is the terminal electron acceptor of the electrons carried by NADH?
A)NAD+
B)FAD
C)O2
D)H2O
E)none of the above
A)NAD+
B)FAD
C)O2
D)H2O
E)none of the above

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
16
The glycolytic reaction
fructose-6-phosphate + ATP fructose-1,6-bisphosphate + ADP
Has an ATP-coupling coefficient of _____ while the gluconeogenic reaction
Fructose-1,6-bisphosphate + H2O fructose-6-phosphate + Pi
Has an ATP-coupling coefficient of _____.
A)1;0
B)-1;0
C)1;-1
D)-1;1
E)none of the above
fructose-6-phosphate + ATP fructose-1,6-bisphosphate + ADP
Has an ATP-coupling coefficient of _____ while the gluconeogenic reaction
Fructose-1,6-bisphosphate + H2O fructose-6-phosphate + Pi
Has an ATP-coupling coefficient of _____.
A)1;0
B)-1;0
C)1;-1
D)-1;1
E)none of the above

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
17
________ is commonly used as an oxidizing agent in catabolic pathways while ________ is commonly used as a reducing agent in anabolic pathways.
A)NAD+;NADH
B)NAD+;FADH2
C)NADP+;NADH
D)FAD;FADH2
E)NAD+;NADPH
A)NAD+;NADH
B)NAD+;FADH2
C)NADP+;NADH
D)FAD;FADH2
E)NAD+;NADPH

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following types of reactions best describes the formation of a peptide bond?
A)nucleophilic substitution
B)nucleophilic addition
C)nucleophilic acyl substitution
D)elimination
E)carbonyl condensation
A)nucleophilic substitution
B)nucleophilic addition
C)nucleophilic acyl substitution
D)elimination
E)carbonyl condensation

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
19
What is the oxidant in the following reaction? 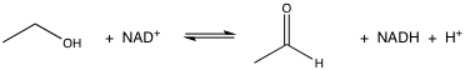
A)ethanol
B)NAD+
C)acetaldehyde
D)NADH
E)H+

A)ethanol
B)NAD+
C)acetaldehyde
D)NADH
E)H+

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
20
The phosphorylation of creatine (Cr)by ATP forming creatine phosphate (Cr-P)is rather endergonic ( G°´ = + 12.6 kJ/mol).Which of the following explains how this reaction proceeds in a typical muscle cell?
A)in the cytosol,[ATP] is high and [Cr] is high allowing the reaction to proceed
B)in the mitochondria,[ATP] is high and [Cr-P] is low allowing the reaction to proceed
C)Cr-P is rapidly transported across the cellular membrane allowing for high levels of Cr-P in the cytosol
D)in the mitochondria,[ADP] is high allowing phosphorylation of Cr to occur
E)none of the above
A)in the cytosol,[ATP] is high and [Cr] is high allowing the reaction to proceed
B)in the mitochondria,[ATP] is high and [Cr-P] is low allowing the reaction to proceed
C)Cr-P is rapidly transported across the cellular membrane allowing for high levels of Cr-P in the cytosol
D)in the mitochondria,[ADP] is high allowing phosphorylation of Cr to occur
E)none of the above

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
21
Pyruvic acid has the formula C3H4O3.Write the reaction for its complete combustion.What is its respiratory quotient? Does this mean that it is more oxidized or less oxidized than glucose?

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
22
Complete oxidation of glucose by burning in a calorimeter gives a G°´ of -2870 kJ/mol.If we assume this to be the maximum energy that can be extracted from glucose,what is the percent of energy that is captured in a cell given that about 32 ATP can be made from a single molecule of glucose? G°´ for ATP hydrolysis is -30.5 kJ/mol while G for ATP hydrolysis in a cell is about -51 kJ/mol.

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
23
Show the reduction of pyruvate by NADH.Since NADH reduces molecules by a hydride reduction,what type of reaction is pyruvate undergoing? 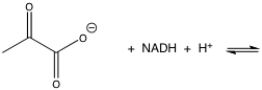


Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
24
Many enzyme-catalyzed reactions that transfer a phosphate from one molecule to another utilize a histidine residue in the active site of the enzyme.The histidine participates in covalent catalysis by performing a nucleophilic attack on a phosphate,forming an intermediate phosphohistidine (the phosphate covalently attached to the histidine residue).Using ATP as the phosphate donor,show the reaction to form the phosphohistidine intermediate-you do not have to show arrow pushing.

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck


