Deck 3: An Introduction to Organic Compounds: Nomenclature, physical Properties, and Structure
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/136
Play
Full screen (f)
Deck 3: An Introduction to Organic Compounds: Nomenclature, physical Properties, and Structure
1
Identify the number of tertiary carbons in the following structure. 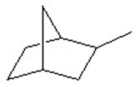
A)2
B)3
C)4
D)5
E)6

A)2
B)3
C)4
D)5
E)6
3
2
Give structures for the three isomers with molecular formula C5H12 and provide the common name of each.
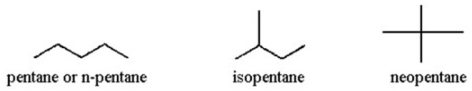
3
Give the IUPAC name for the following structure. 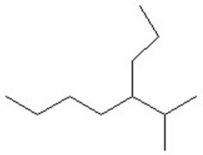
A)2-methyl-3-ethylheptane
B)3-ethyl-2-methylheptane
C)5-Isopropyloctane
D)4-Isopropyloctane
E)2-methyl-3-propylheptane

A)2-methyl-3-ethylheptane
B)3-ethyl-2-methylheptane
C)5-Isopropyloctane
D)4-Isopropyloctane
E)2-methyl-3-propylheptane
4-Isopropyloctane
4
There is something wrong with the following name.Write the structure and correct the name: 2-ethylpropane.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
5
Provide an acceptable name for the alkane shown below. 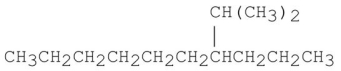
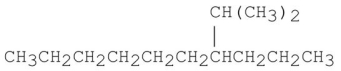

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
6
Draw an acceptable structure for 3-ethyl-3-methylhexane.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
7
Provide an acceptable name for the alkane shown below. 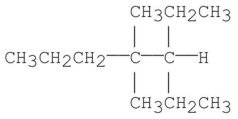


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
8
Provide an acceptable name for the alkane shown below. 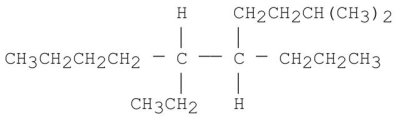


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
9
Provide an acceptable name for the alkane shown below. 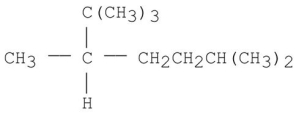


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
10
Draw an acceptable structure for 4-t-butyloctane.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
11
Provide an acceptable name for the alkane shown below.
CH3CH2CH2CH2CH2CH3
CH3CH2CH2CH2CH2CH3

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is sec-butyl alcohol?
A)CH3CH2CH2CH2OH
B)CH3CH(OH)CH2CH3
C)(CH3)2CHCH2OH
D)(CH3)2CHOH
E)(CH3)2CHOCH3
A)CH3CH2CH2CH2OH
B)CH3CH(OH)CH2CH3
C)(CH3)2CHCH2OH
D)(CH3)2CHOH
E)(CH3)2CHOCH3

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
13
There are 8 isomers that have the molecular formula C5H11Br.How many of these are tertiary alkyl bromides?
A)0
B)1
C)2
D)3
E)8
A)0
B)1
C)2
D)3
E)8

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
14
Draw an acceptable structure for 6-ethyl-2,6,7-trimethyl-5-propylnonane.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
15
What is the common name for the following structure? 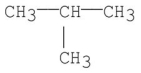
A)isobutane
B)isopropylmethane
C)t-Butane
D)n-Butane
E)sec-Butane

A)isobutane
B)isopropylmethane
C)t-Butane
D)n-Butane
E)sec-Butane

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
16
Draw an acceptable structure for 4-isopropyl-2-methylheptane.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
17
Provide an acceptable name for the alkane shown below. 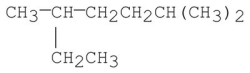
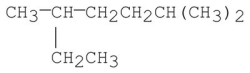

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
18
Provide an acceptable name for the alkane shown below. 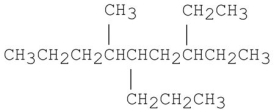


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
19
Provide an acceptable name for the alkane shown below. 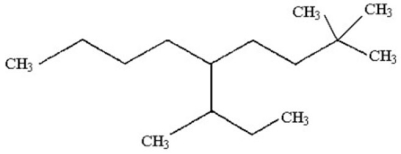


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is a tertiary amine?
A)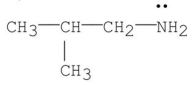
B)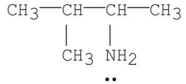
C)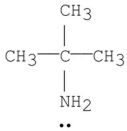
D)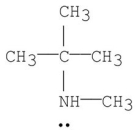
E)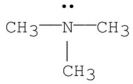
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
21
Provide an acceptable name for the compound shown below. 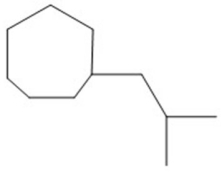


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
22
Give the systematic name of the alkane shown below. 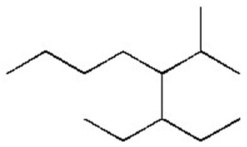


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
23
Provide the IUPAC name of the compound. 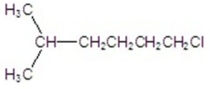
A)2-methylheptane
B)1-chloro-5-methylhexane
C)6-chloro-2-methylhexane
D)1,1-dimethyl-5-chloropentane

A)2-methylheptane
B)1-chloro-5-methylhexane
C)6-chloro-2-methylhexane
D)1,1-dimethyl-5-chloropentane

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
24
Provide the common name of the compound. 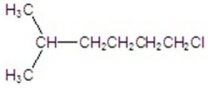
A)neoheptyl chloride
B)sec-heptyl chloride
C)isoheptyl chloride
D)tert-heptyl chloride
E)n-heptyl chloride

A)neoheptyl chloride
B)sec-heptyl chloride
C)isoheptyl chloride
D)tert-heptyl chloride
E)n-heptyl chloride

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
25
Provide an acceptable name for the compound shown below. 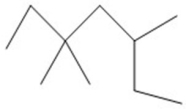


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
26
Provide an acceptable name for the compound shown below. 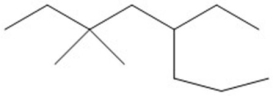


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
27
Provide an acceptable name for the compound shown below. 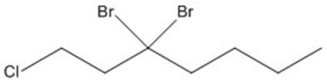


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
28
Give the systematic name of the cycloalkane shown below. 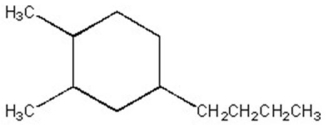


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
29
Draw all possible constitutional isomers for C2H6O and give common names for each structure.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
30
Name the compound. 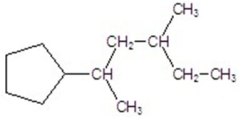
A)2-cyclopentyl-4-methylhexane
B)5-cyclopentyl-3-methylhexane
C)1-cyclopentyl-1,3-dimethylpentane
D)2-cyclopentyl-4-methylheptane
E)5-cyclopentyl-3-methylheptane

A)2-cyclopentyl-4-methylhexane
B)5-cyclopentyl-3-methylhexane
C)1-cyclopentyl-1,3-dimethylpentane
D)2-cyclopentyl-4-methylheptane
E)5-cyclopentyl-3-methylheptane

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
31
Draw an acceptable structure for sec-butylcyclopentane.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
32
What is the common name for the following structure? 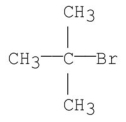
A)isobutyl bromide
B)t-Butyl bromide
C)neobutyl bromide
D)sec-Butyl bromide
E)isopropyl methyl bromide

A)isobutyl bromide
B)t-Butyl bromide
C)neobutyl bromide
D)sec-Butyl bromide
E)isopropyl methyl bromide

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
33
Give the formula. 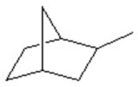
A)C8H8
B)C8H10
C)C8H12
D)C8H14
E)C8H16

A)C8H8
B)C8H10
C)C8H12
D)C8H14
E)C8H16

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
34
Determine the number of hydrogens in limonene. 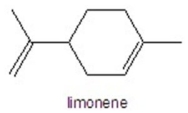
A)8
B)10
C)12
D)16
E)18

A)8
B)10
C)12
D)16
E)18

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is diisopropyl ether?
A)CH3CH2CH2-O-CH2CH2CH3
B)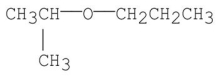
C)CH3CH2CH2OCH(CH3)2
D)(CH3)3COC(CH3)3
E)(CH3)2CHOCH(CH3)2
A)CH3CH2CH2-O-CH2CH2CH3
B)

C)CH3CH2CH2OCH(CH3)2
D)(CH3)3COC(CH3)3
E)(CH3)2CHOCH(CH3)2

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
36
Provide the systematic name of the compound shown. 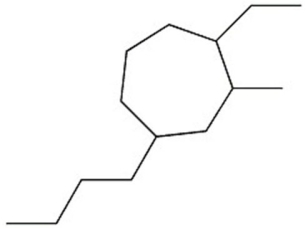


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
37
Give the systematic name of the alkane shown below. 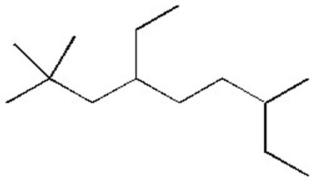


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
38
Draw all ethers with molecular formula C4H10O.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
39
Name the compound. 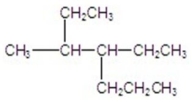
A)2-ethyl-3-propylpentane
B)2,3-diethylhexane
C)4-ethyl-5-methylheptane
D)4-methyl-3-ethylheptane
E)4-ethyl-3-methylheptane

A)2-ethyl-3-propylpentane
B)2,3-diethylhexane
C)4-ethyl-5-methylheptane
D)4-methyl-3-ethylheptane
E)4-ethyl-3-methylheptane

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
40
Give the IUPAC name for the following compound. 
A)1-chloro-2-methylcyclohexane
B)1-methyl-2-chlorocyclohexane
C)1-chloro-5-methylcyclohexane
D)1-methyl-5-chlorocyclohexane
E)1,2-chloromethylcyclohexane

A)1-chloro-2-methylcyclohexane
B)1-methyl-2-chlorocyclohexane
C)1-chloro-5-methylcyclohexane
D)1-methyl-5-chlorocyclohexane
E)1,2-chloromethylcyclohexane

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
41
Provide an acceptable name for the compound shown below. 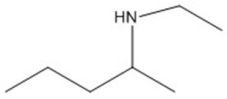


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
42
Identify the compound with the highest boiling point.
A)CH3CH2OCH2CH3
B)CH3CH2CH2CH2OH
C)CH3CH2CH2CH2NH2
D)CH3CH2CH2CH2Cl
E)CH3CH2CH2CH2Br
A)CH3CH2OCH2CH3
B)CH3CH2CH2CH2OH
C)CH3CH2CH2CH2NH2
D)CH3CH2CH2CH2Cl
E)CH3CH2CH2CH2Br

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following will have the lowest boiling point?
A)CH3Cl
B)CH4
C)CH2Cl2
D)CHCl3
E)CCl4
A)CH3Cl
B)CH4
C)CH2Cl2
D)CHCl3
E)CCl4

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
44
Identify the compound(s)that do not have hydrogen bonding.You may choose more than one answer.
A)(CH3CH2CH2NHCH3)
B)(CH3CH2CH2CH2Cl)
C)(CH3CH2CH2CH2NH2)
D)(CH3CH2CH2CH2OH)
E)(CH3CH2CH2OCH3)
A)(CH3CH2CH2NHCH3)
B)(CH3CH2CH2CH2Cl)
C)(CH3CH2CH2CH2NH2)
D)(CH3CH2CH2CH2OH)
E)(CH3CH2CH2OCH3)

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
45
Draw the structure of N-ethyl-5-methyl-3-hexanamine.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
46
Identify the compound with the highest bond angle.
A)the C-O-C bond in an ether
B)the C-N-C bond in a secondary amine
C)the C-N-C bond in a quaternary amine
D)the C-O-C bond in an alcohol
E)They are all equal.
A)the C-O-C bond in an ether
B)the C-N-C bond in a secondary amine
C)the C-N-C bond in a quaternary amine
D)the C-O-C bond in an alcohol
E)They are all equal.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following compounds does not have the molecular formula C6H14O?
A)1-hexanol
B)2-hexanol
C)3-methyl-2-pentanol
D)3-methyl-3-pentanol
E)cyclohexanol
A)1-hexanol
B)2-hexanol
C)3-methyl-2-pentanol
D)3-methyl-3-pentanol
E)cyclohexanol

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
48
Fluorine is more electronegative than chlorine yet the carbon-fluorine bond in CH3-F is shorter than CH3-Cl.Explain.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
49
Give the IUPAC name for the following structure. 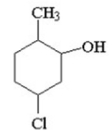
A)3-chloro-6-methylcyclohexanol
B)2-methyl-5-chlorocyclohexanol
C)1-chloro-4-methylcyclohexanol
D)5-chloro-2-methylcyclohexanol
E)2-methyl-3-chlorocyclohexanol

A)3-chloro-6-methylcyclohexanol
B)2-methyl-5-chlorocyclohexanol
C)1-chloro-4-methylcyclohexanol
D)5-chloro-2-methylcyclohexanol
E)2-methyl-3-chlorocyclohexanol

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
50
Name the structure. 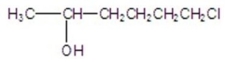
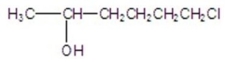

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
51
Provide the IUPAC name of the compound. 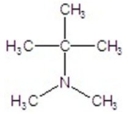
A)N,N,1,1-tetramethylethanamine
B)N,N-dimethyl-2-butanamine
C)N,N,2-trimethyl-1-propanamine
D)N,N,2-trimethylpropanamine
E)N,N,2-trimethyl-2-propanamine

A)N,N,1,1-tetramethylethanamine
B)N,N-dimethyl-2-butanamine
C)N,N,2-trimethyl-1-propanamine
D)N,N,2-trimethylpropanamine
E)N,N,2-trimethyl-2-propanamine

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
52
Classify the following amines as primary,secondary,or tertiary. 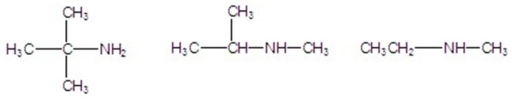


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
53
Provide the IUPAC name of the compound.
H2NCH2CH2CH2CH2OH
H2NCH2CH2CH2CH2OH

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the compounds below will form hydrogen bonds between its molecules?
A)CH3CH2CH2F
B)CH3CH2CH2CH3
C)(CH3)3N
D)CH3CH2OCH3
E)CH3NHCH2CH3
A)CH3CH2CH2F
B)CH3CH2CH2CH3
C)(CH3)3N
D)CH3CH2OCH3
E)CH3NHCH2CH3

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
55
Give the structure of isopentyl alcohol.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
56
Draw the structure of 3-chloro-N-ethyl-2-hexanamine.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
57
Provide the systematic name of the compound shown. 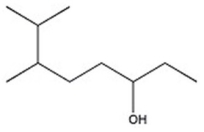


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
58
Provide an acceptable name for the compound shown below. 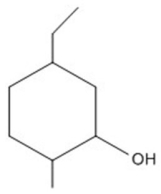


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
59
Give the structure of tetramethylammonium chloride.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
60
Provide the common name of the compound. 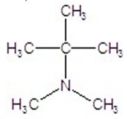
A)tert-butyldimethylamine
B)isobutyldimethylamine
C)neobutyldimethylamine
D)sec-butyldimethylamine
E)n-butyldimethylamine

A)tert-butyldimethylamine
B)isobutyldimethylamine
C)neobutyldimethylamine
D)sec-butyldimethylamine
E)n-butyldimethylamine

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following has the greatest solubility in CH3CH2CH2CH3?
A)CH3OH
B)CH3O- Na+
C)CH3NH2
D)CH3OCH3
E)(CH3)3CH
A)CH3OH
B)CH3O- Na+
C)CH3NH2
D)CH3OCH3
E)(CH3)3CH

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
62
Assuming roughly equivalent molecular weights,which of the following would have the highest boiling point?
A)a tertiary amine
B)a quaternary ammonium salt
C)an alcohol
D)an ether
E)an alkyl chloride
A)a tertiary amine
B)a quaternary ammonium salt
C)an alcohol
D)an ether
E)an alkyl chloride

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
63
The eclipsed and staggered forms of ethane are said to differ in
A)molecular formula.
B)configuration.
C)conformation.
D)constitution.
E)structure.
A)molecular formula.
B)configuration.
C)conformation.
D)constitution.
E)structure.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
64
Would you expect sodium chloride (NaCl)to be highly soluble in the organic solvent hexane (CH3CH2CH2CH2CH2CH3)? Briefly explain your answer.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
65
Which intermolecular force is primarily responsible for the interactions among alkane molecules?

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
66
Primary and secondary amines exhibit hydrogen bonding; tertiary amines do not.Explain.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following is the staggered conformation for rotation about the C1-C2 bond in the following structure? 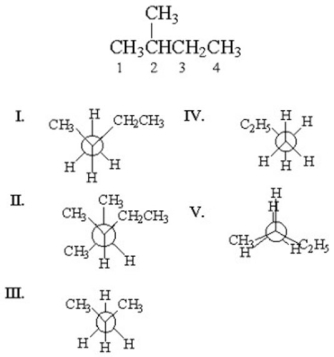
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following is the most soluble in H2O?
A)CH3OCH3
B)CH3CH2OH
C)CH3CH2Cl
D)CH3CH2CH3
E)CH3CHO
A)CH3OCH3
B)CH3CH2OH
C)CH3CH2Cl
D)CH3CH2CH3
E)CH3CHO

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
69
Which compound is more soluble in water? Briefly explain your choice.
CH3OCH3 or CH3CH2OH
CH3OCH3 or CH3CH2OH

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
70
What is polarizability and how is it related to the size of an atom?

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
71
Consider the three isomeric alkanes n-hexane,2,3-dimethylbutane,and 2-methylpentane.Which of the following correctly lists these compounds in order of increasing boiling point?
A)2,3-dimethylbutane < 2-methylpentane < n-hexane
B)2-methylpentane < n-hexane < 2,3-dimethylbutane
C)2-methylpentane < 2,3-dimethylbutane < n-hexane
D)n-hexane < 2-methylpentane < 2,3-dimethylbutane
E)n-hexane < 2,3-dimethylbutane < 2-methylpentane
A)2,3-dimethylbutane < 2-methylpentane < n-hexane
B)2-methylpentane < n-hexane < 2,3-dimethylbutane
C)2-methylpentane < 2,3-dimethylbutane < n-hexane
D)n-hexane < 2-methylpentane < 2,3-dimethylbutane
E)n-hexane < 2,3-dimethylbutane < 2-methylpentane

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
72
Explain why trimethylamine,(CH3)3N:,has a considerably lower boiling point than propylamine CH3CH2CH2NH2,even though both compounds have the same molecular formula.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following has the lowest boiling point?
A)CH3CH2CH2CH2CH2CH3
B)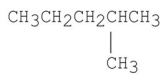
C)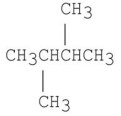
D)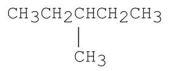
E)CH3CH2CH2CH2CH2CH2CH3
A)CH3CH2CH2CH2CH2CH3
B)
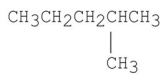
C)

D)
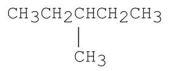
E)CH3CH2CH2CH2CH2CH2CH3

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
74
Which compound is more soluble in water? Briefly explain your choice.
(CH3)2NH or CH3CH2CH3
(CH3)2NH or CH3CH2CH3

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following has the greatest van der Waal's interaction between molecules of the same kind?
A)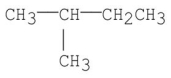
B)CH3CH2CH2CH3
C)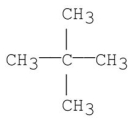
D)CH3CH2CH2CH2CH3
E)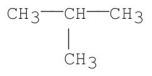
A)

B)CH3CH2CH2CH3
C)

D)CH3CH2CH2CH2CH3
E)


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
76
What is the strongest intermolecular force present in liquid ethanol?
A)induced dipole-induced dipole
B)dipole-dipole,specifically hydrogen bonding
C)dipole-dipole,but not hydrogen bonding
D)ion-dipole
E)ion-ion
A)induced dipole-induced dipole
B)dipole-dipole,specifically hydrogen bonding
C)dipole-dipole,but not hydrogen bonding
D)ion-dipole
E)ion-ion

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following would have the highest boiling point?
A)CH3CH2-O-CH2CH2-O-CH3
B)CH3-O-CH2CH2CH2-O-CH3
C)HO-CH2CH2CH2CH2-OH
D)CH3CH2-O-CH2-O-CH2CH3
E)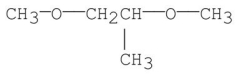
A)CH3CH2-O-CH2CH2-O-CH3
B)CH3-O-CH2CH2CH2-O-CH3
C)HO-CH2CH2CH2CH2-OH
D)CH3CH2-O-CH2-O-CH2CH3
E)
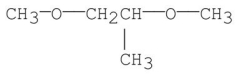

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the molecules below has the higher boiling point? Briefly explain your choice.
CH3CH2CH2OH or CH3CH2OCH3
CH3CH2CH2OH or CH3CH2OCH3

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
79
Arrange the following amines in order of increasing boiling point,lowest bp to highest bp: (CH3)2CHCH2CH2NH2,(CH3)2CHN(CH3)2,and (CH3)2CHCH2NHCH3.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
80
Explain why the molecule shown below has a lower boiling point than CH3CH2CH2CH3. 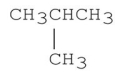


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck


