Deck 14: Nmr Spectroscopy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/122
Play
Full screen (f)
Deck 14: Nmr Spectroscopy
1
Determine the number of signals for isopentylamine in the 1H NMR spectrum.
A)2
B)3
C)4
D)5
E)6
A)2
B)3
C)4
D)5
E)6
5
2
How many signals would you expect to see in the 1H NMR spectrum of the following compound? CH3CH2CH2CH3
A)1
B)3
C)2
D)4
E)6
A)1
B)3
C)2
D)4
E)6
2
3
How many proton NMR singlets will 2-bromo-3-methyl-2-butene exhibit?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5
3
4
If the frequency for flipping a 1H nucleus at an applied field of 1.4092 Tesla is 60 MHz,what would be the applied magnetic field if the frequency is 360 MHz?
A)8)4552 Tesla
B)0)2349 Tesla
C)1)4092 Tesla
D)4)2577 Tesla
E)3)0439 Tesla
A)8)4552 Tesla
B)0)2349 Tesla
C)1)4092 Tesla
D)4)2577 Tesla
E)3)0439 Tesla

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
5
How many signals would you expect to see in the 1H NMR spectrum of the following compound? 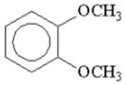
A)5
B)4
C)2
D)3
E)1

A)5
B)4
C)2
D)3
E)1

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
6
How many signals would you expect to see in the 1H NMR spectrum of the following compound? 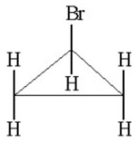
A)1
B)2
C)4
D)5
E)3

A)1
B)2
C)4
D)5
E)3

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
7
Determine the number of signals for 4-methyl-1-propylbenzene in the 1H NMR spectrum.
A)4
B)5
C)6
D)7
E)8
A)4
B)5
C)6
D)7
E)8

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
8
How many signals would you expect to see in the 1H NMR spectrum of the following compound? 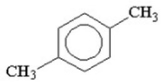
A)6
B)5
C)4
D)3
E)2

A)6
B)5
C)4
D)3
E)2

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
9
How many signals would you expect to see in the 1H NMR spectrum of the following compound? ClCH2CH2Cl
A)5
B)4
C)3
D)2
E)1
A)5
B)4
C)3
D)2
E)1

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
10
How many signals would you expect to see in the 1H NMR spectrum of the following compound? 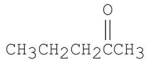
A)2
B)4
C)3
D)5
E)6
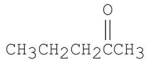
A)2
B)4
C)3
D)5
E)6

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
11
How many signals would you expect to see in the 1H NMR spectrum of the following compound? 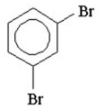
A)1
B)2
C)3
D)4
E)5

A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following spectroscopic techniques uses the lowest energy of the electromagnetic radiation spectrum?
A)UV
B)visible
C)IR
D)X-ray
E)NMR
A)UV
B)visible
C)IR
D)X-ray
E)NMR

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
13
How many signals would you expect to see in the 1H NMR spectrum of the following compound? 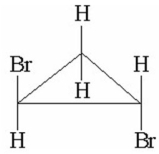
A)2
B)5
C)1
D)4
E)3

A)2
B)5
C)1
D)4
E)3

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following electromagnetic wave types is used in nuclear magnetic resonance spectroscopy?
A)X-ray
B)infrared
C)visible
D)radio
E)ultraviolet
A)X-ray
B)infrared
C)visible
D)radio
E)ultraviolet

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
15
If a chemical shift of an NMR signal is 7.2 ppm measured in a 60 MHz NMR spectrometer,how many Hz would this signal be from the TMS signal?
A)8)3 Hz
B)432 Hz
C)0)12 Hz
D)72 Hz
E)60 Hz
A)8)3 Hz
B)432 Hz
C)0)12 Hz
D)72 Hz
E)60 Hz

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
16
How many signals would you expect to see in the 1H NMR spectrum of the following compound? 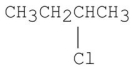
A)1
B)2
C)4
D)3
E)6
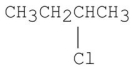
A)1
B)2
C)4
D)3
E)6

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
17
1H nuclei located near electronegative atoms tend to be ________ relative to 1H nuclei which are not.
A)shielded
B)deshielded
C)resonanced
D)split
E)none of the above
A)shielded
B)deshielded
C)resonanced
D)split
E)none of the above

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
18
How many signals would you expect to see in the 1H NMR spectrum of the following compound? 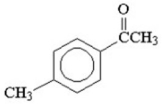
A)6
B)3
C)5
D)4
E)2

A)6
B)3
C)5
D)4
E)2

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
19
How many signals would you expect to see in the 1H NMR spectrum of the following compound? 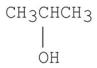
A)7
B)3
C)4
D)2
E)8
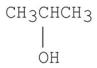
A)7
B)3
C)4
D)2
E)8

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
20
Why is Fourier transform NMR spectroscopy preferred over continuous wave as a technique for 13C NMR?

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following protons gives an NMR signal with the highest chemical shift value (farthest downfield)? (CH3)2CH-O-CH2CH2CH3
1 2 3 4 5
A)1
B)2
C)3
D)4
E)5
1 2 3 4 5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following protons gives an NMR signal with the lowest chemical shift value (farthest upfield)? F-CH2CH2CH2CH2CH2-Br
1 2 3 4 5
A)1
B)2
C)3
D)4
E)5
1 2 3 4 5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
23
How many distinct triplets would you expect in the 1H NMR of the compound below? 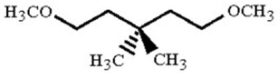
A)0
B)2
C)4
D)6
E)8

A)0
B)2
C)4
D)6
E)8

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
24
What splitting pattern is observed in the proton NMR spectrum for the indicated hydrogens? 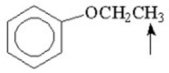
A)singlet
B)doublet
C)triplet
D)quartet
E)septet

A)singlet
B)doublet
C)triplet
D)quartet
E)septet

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
25
What is the ratio of the protons in the following compound? 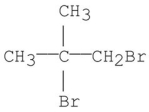
A)3:3:2
B)3:2
C)6:2:1
D)3:1
E)3:2:1

A)3:3:2
B)3:2
C)6:2:1
D)3:1
E)3:2:1

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following compounds gives the highest chemical shift value (farthest downfield)in the NMR spectrum? 
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
27
What is the ratio of the protons in the following compound? 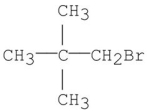
A)3:3:3:2
B)6:3:2
C)9:2
D)3:2
E)6:2

A)3:3:3:2
B)6:3:2
C)9:2
D)3:2
E)6:2

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
28
Describe the difference between a ketone and an aldehyde in the 1H NMR spectrum.
A)An aldehyde has a signal at 9.0 - 10.0 ppm.
B)An aldehyde has a signal at 2.1 ppm.
C)An aldehyde has a signal at 5.0 ppm.
D)An aldehyde has a signal at 3.5 ppm.
E)An aldehyde has a signal at 8.0 ppm.
A)An aldehyde has a signal at 9.0 - 10.0 ppm.
B)An aldehyde has a signal at 2.1 ppm.
C)An aldehyde has a signal at 5.0 ppm.
D)An aldehyde has a signal at 3.5 ppm.
E)An aldehyde has a signal at 8.0 ppm.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
29
H-H coupling is observed in the 1H NMR spectrum of CH3CH2OCH3 and the signal from the methylene H's does not appear as a single peak.How does this signal appear? Explain in detail what is occurring.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
30
Give the splitting pattern for each proton in the 1H NMR spectrum. 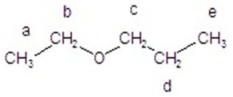
A)a = triplet; b = doublet; c = doublet; d = multiplet; e = triplet
B)a = doublet; b = triplet; c = doublet; d = multiplet; e = doublet
C)a = triplet; b = quartet; c = triplet; d = multiplet; e = triplet
D)a = singlet; b = doublet; c = singlet; d = multiplet; e = singlet
E)a = triplet; b = quartet; c= triplet; d = multiplet; e = doublet

A)a = triplet; b = doublet; c = doublet; d = multiplet; e = triplet
B)a = doublet; b = triplet; c = doublet; d = multiplet; e = doublet
C)a = triplet; b = quartet; c = triplet; d = multiplet; e = triplet
D)a = singlet; b = doublet; c = singlet; d = multiplet; e = singlet
E)a = triplet; b = quartet; c= triplet; d = multiplet; e = doublet

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
31
If two signals differ by 3 ppm,how do they differ in Hertz in a 60 MHz spectrometer?
A)20 Hz
B)300 Hz
C)360 Hz
D)180 Hz
E)90 Hz
A)20 Hz
B)300 Hz
C)360 Hz
D)180 Hz
E)90 Hz

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
32
Predict the integration for each proton in the 1H NMR spectrum. 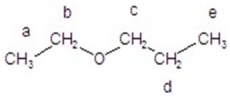
A)a = 2H; b = 3H; c = 2H; d = 3H; e = 2H
B)a = 2H; b = 3H; c = 2H; d = 5H; e = 2H
C)a = 5H; b = 5H; c = 7H; d = 7H; e = 7H
D)a = 3H; b = 2H; c = 2H; d = 2H; e = 3H
E)a = 3H; b = 2H; c = 2H; d = 3H; e = 2H

A)a = 2H; b = 3H; c = 2H; d = 3H; e = 2H
B)a = 2H; b = 3H; c = 2H; d = 5H; e = 2H
C)a = 5H; b = 5H; c = 7H; d = 7H; e = 7H
D)a = 3H; b = 2H; c = 2H; d = 2H; e = 3H
E)a = 3H; b = 2H; c = 2H; d = 3H; e = 2H

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following methyl groups will exhibit the most downfield (highest)chemical shift in 1H NMR spectroscopy? 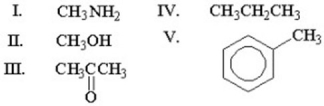
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
34
What splitting pattern is observed in the proton NMR spectrum for the indicated hydrogens? CH3OCH2CH2OCH3
↑
A)singlet
B)doublet
C)triplet
D)quartet
E)septet
↑
A)singlet
B)doublet
C)triplet
D)quartet
E)septet

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
35
________ is commonly used as an internal reference in NMR spectroscopy; its signal is assigned δ=0 in 1H and 13C NMR spectroscopy.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
36
What splitting pattern is observed in the proton NMR spectrum for the indicated hydrogens? 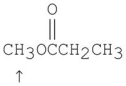
A)singlet
B)doublet
C)triplet
D)quartet
E)septet

A)singlet
B)doublet
C)triplet
D)quartet
E)septet

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following protons gives an NMR signal with the lowest chemical shift value (farthest upfield)? 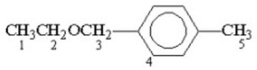
A)1
B)2
C)3
D)4
E)5

A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
38
Using a 60 MHz spectrometer,the protons in dichloromethane appear at 5.30 ppm.When the same sample is placed in a 100 MHz instrument,where does the signal appear?
A)8)33 ppm
B)3)18 ppm
C)5)30 ppm
D)cannot be determined from information given
A)8)33 ppm
B)3)18 ppm
C)5)30 ppm
D)cannot be determined from information given

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following protons gives an NMR signal with the highest chemical shift value (farthest downfield)? F-CH2CH2CH2CH2CH2-Br
1 2 3 4 5
A)1
B)2
C)3
D)4
E)5
1 2 3 4 5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
40
What splitting pattern is observed in the proton NMR spectrum for the indicated hydrogens? 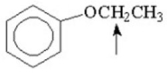
A)singlet
B)doublet
C)triplet
D)quartet
E)septet

A)singlet
B)doublet
C)triplet
D)quartet
E)septet

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
41
Deduce the identity of the following compound from the spectral data given.
C9H10O2: 13C NMR,δ 18.06 (quartet),45.40 (doublet),127.32 (doublet),127.55 (doublet),128.61 (doublet),139.70 (singlet),180.98 (singlet); IR,broad 3500-2800,1708 cm-1
C9H10O2: 13C NMR,δ 18.06 (quartet),45.40 (doublet),127.32 (doublet),127.55 (doublet),128.61 (doublet),139.70 (singlet),180.98 (singlet); IR,broad 3500-2800,1708 cm-1

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the compounds below most closely matches the following 1H NMR data:
7.6 (2H,d),7.3 (2H,d),3.5 (3H,s),2.2 (3H,s)?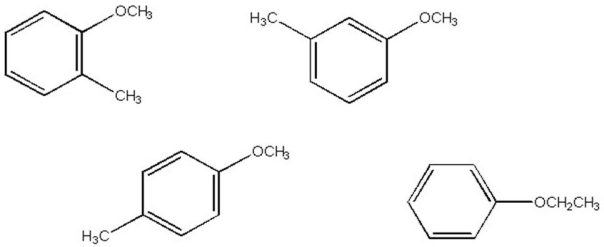
7.6 (2H,d),7.3 (2H,d),3.5 (3H,s),2.2 (3H,s)?


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
43
Predict the number of signals expected,their splitting,and their relative area in the 1H NMR spectrum of CH3CH2OCH3.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
44
Deduce the identity of the following compound from the spectral data given.
C4H8O2: 1H NMR,δ 1.23 (3H,triplet),2.00 (3H,singlet),4.02 (2H,quartet); IR,2980,1740 cm-1
C4H8O2: 1H NMR,δ 1.23 (3H,triplet),2.00 (3H,singlet),4.02 (2H,quartet); IR,2980,1740 cm-1

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
45
An unknown compound,C3H5Cl3,gave the following proton NMR data:
Doublet at 1.70 ppm (3H)
Multiplet at 4.32 ppm (1H)
Doublet at 5.85 ppm (1H)
What is the structure of the compound?
Doublet at 1.70 ppm (3H)
Multiplet at 4.32 ppm (1H)
Doublet at 5.85 ppm (1H)
What is the structure of the compound?

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
46
Predict the number of signals expected,their splitting,and their relative area in the 1H NMR spectrum of (CH3)3CCHO.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
47
An unknown compound,C4H10O,gave the following proton NMR data:
Triplet at 1.13 ppm
Quartet at 3.38 ppm
What is the structure of the compound?
Triplet at 1.13 ppm
Quartet at 3.38 ppm
What is the structure of the compound?

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
48
Deduce the identity of the following compound from the 1H NMR data given.
C4H7BrO: δ 2.2 (3H,singlet),3.5 (2H,triplet),4.5 (2H,triplet)
C4H7BrO: δ 2.2 (3H,singlet),3.5 (2H,triplet),4.5 (2H,triplet)

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following compounds has a triplet at 9.8 in its 1H NMR spectrum? 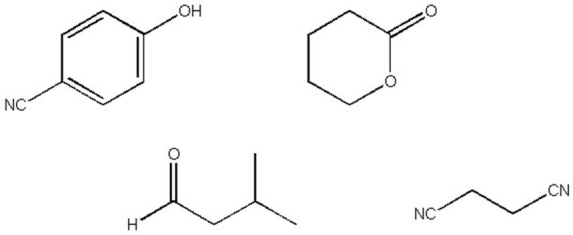


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
50
An unknown compound,C4H8Br2,gave the following proton NMR data:
Singlet at 1.97 ppm (6H)
Singlet at 3.89 ppm (2H)
What is the compound?
Singlet at 1.97 ppm (6H)
Singlet at 3.89 ppm (2H)
What is the compound?

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
51
Deduce the identity of the compound from the data provided.
C10H14O: IR (cm-1): 3200-3500 (broad),3050,2950,1610
1H NMR(δ): 1.0 (s,6H),2.0 (s,3H),2.8 (broad s,1H),
7.3 (d,2H),7.6 (d,2H)
C10H14O: IR (cm-1): 3200-3500 (broad),3050,2950,1610
1H NMR(δ): 1.0 (s,6H),2.0 (s,3H),2.8 (broad s,1H),
7.3 (d,2H),7.6 (d,2H)

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
52
Deduce the structure from the data given: C7H16O; 1H NMR δ (integration,coupling): 0.9 (9H,s),1.5 (2H,t),3.5 (3H,s),3.8 (2H,t).

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following technique(s)can readily distinguish between:  ?
?
A)NMR
B)IR
C)MS
D)A and B
E)A and C
 ?
?A)NMR
B)IR
C)MS
D)A and B
E)A and C

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
54
An unknown compound,C9H12,gave the following NMR spectrum: Triplet at 1.21 ppm (3H)Singlet at 2.30 ppm (3H)Quartet at 2.60 ppm (2H)Singlet at 7.04 ppm (4H)
What is the structure of the compound?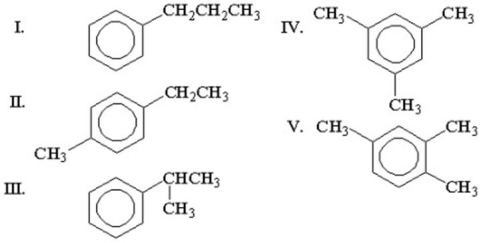
A)I
B)II
C)III
D)IV
E)V
What is the structure of the compound?

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
55
Deduce the identity of the following compound from the spectral data given.
C5H10O: 1H NMR,δ 1.2 (6H,doublet),2.1 (3H,singlet),2.8 (1H,septet); IR,2980,1710 cm-1; MS,m/z 71,
C5H10O: 1H NMR,δ 1.2 (6H,doublet),2.1 (3H,singlet),2.8 (1H,septet); IR,2980,1710 cm-1; MS,m/z 71,

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
56
Deduce the identity of the following compound from the 1H NMR data given.
C8H10O: δ 3.4 (3H,singlet),4.5 (2H,singlet),7.2 (5H,singlet)
C8H10O: δ 3.4 (3H,singlet),4.5 (2H,singlet),7.2 (5H,singlet)

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
57
Give the integration and splitting pattern for the indicated signals in the 1H NMR spectrum. 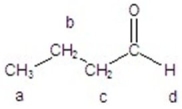
A)a = 3H,triplet; d = 1H,singlet
B)a = 2H,triplet; d = 1H,singlet
C)a = 3H,triplet,d = 1H,singlet
D)a = 2H,triplet; d = 2H,doublet
E)a = 3H,triplet; d = 1H,triplet

A)a = 3H,triplet; d = 1H,singlet
B)a = 2H,triplet; d = 1H,singlet
C)a = 3H,triplet,d = 1H,singlet
D)a = 2H,triplet; d = 2H,doublet
E)a = 3H,triplet; d = 1H,triplet

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
58
Predict the number of signals expected,their splitting,and their relative area in the 1H NMR spectrum of 1,2-dichloroethane (ClCH2CH2Cl).

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
59
Which compound has a 1H NMR spectrum consisting of the following peaks: 0.9 (6H,d),1.0 (3H,t),2.2 (2H,q),and 4.0 (1H,septet)?
A)(CH3)2CHCH2O2CCH3
B)(CH3)2CHCH2CO2CH3
C)(CH3)2CHO2CCH2CH3
D)(CH3)2CHCO2CH2CH3
E)(CH3)2CHOCH2CH3
A)(CH3)2CHCH2O2CCH3
B)(CH3)2CHCH2CO2CH3
C)(CH3)2CHO2CCH2CH3
D)(CH3)2CHCO2CH2CH3
E)(CH3)2CHOCH2CH3

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
60
Deduce the identity of the following compound from the spectral data given.
C7H10O2: 1H NMR,δ 1.16 (3H,singlet),2.21 (2H,singlet); 13C NMR,δ 216.25 (singlet),52.57 (singlet),34.51 (triplet),20.22 (quartet)
C7H10O2: 1H NMR,δ 1.16 (3H,singlet),2.21 (2H,singlet); 13C NMR,δ 216.25 (singlet),52.57 (singlet),34.51 (triplet),20.22 (quartet)

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
61
What multiplicities are observed in the spin coupled 13C NMR spectrum of 2,3-dimethyl-2-butene?

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
62
Can alkenes and aromatics be easily distinguished from each other in an 13C NMR spectrum?

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
63
How might the proton spectrum of ultrapure dimethylamine,(CH3)2NH,differ from the spectrum of this compound to which D2O has been added?

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
64
Give the structure of a compound that has a formula of C4H7ClO2 and has a triplet (3H,1.1 ppm),quintet (2H,1.2 ppm),triplet (1H,4.4 ppm),and singlet (1H,11.6 ppm)in the 1H NMR spectrum.
A)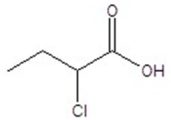
B)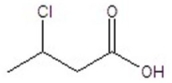
C)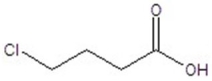
D)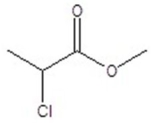
E)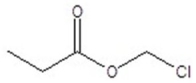
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
65
How might the two trimethylcyclohexane isomers shown below be most readily distinguished using NMR? 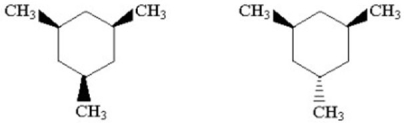
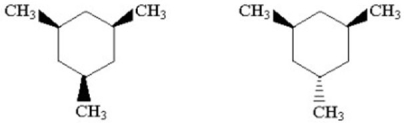

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
66
How many distinct carbon signals are expected in the proton-decoupled 13C NMR spectrum of the compound below? 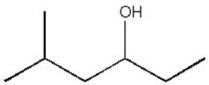


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
67
Give the structure of a compound that has a formula of C5H10O2, and has a triplet (3H,1.1 ppm),sextet (2H,1.8 ppm),triplet (2H,2.3 ppm)and a singlet (3H,3.8 ppm)in the 1H NMR spectrum.
A)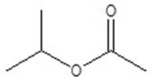
B)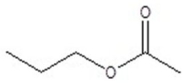
C)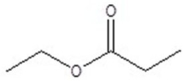
D)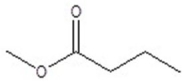
E)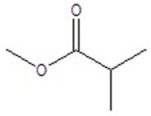
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
68
Deduce the structure from the data given: C8H10O; 13C NMR δ (coupling): 140 (s),128 (d),126 (d),122 (d),60 (t),15 (q).

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following compounds has a signal disappear when D2O is added to it,ethyl alcohol or ethyl chloride?

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
70
Deduce the identity of the compound from the data provided. C5H8O4: IR (cm-1): 2800-3300 (broad),2950,1740
13C NMR (δ,splitting): 17.3 (q),44.3 (s),210.5 (s)
A)HO2CC(CH3)2CO2H
B)CH3O2CCH2CO2CH3
C)HO2CCH2CH2CH2CO2H
D)CH3O2CCH2CH2CO2H
E)CH3O2CCO2CH2CH3
13C NMR (δ,splitting): 17.3 (q),44.3 (s),210.5 (s)
A)HO2CC(CH3)2CO2H
B)CH3O2CCH2CO2CH3
C)HO2CCH2CH2CH2CO2H
D)CH3O2CCH2CH2CO2H
E)CH3O2CCO2CH2CH3

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
71
How many distinct carbon signals are expected in the proton-decoupled 13C NMR spectrum of the compound below? 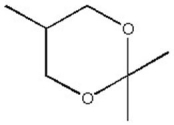


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
72
A compound gave a signal at 203 ppm in the 13C NMR spectrum.How would it be possible to tell if the compound is an aldehyde or a ketone in a proton-coupled 13C NMR spectrum?

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
73
The chair form of cyclohexane has protons in two distinct environments,axial and equatorial.When the proton NMR of cyclohexane is run on a 100 MHz instrument at 23°C,only one signal for the compound is observed.Explain this apparent contradiction.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
74
Deduce the identity of the compound from the data provided. C7H12: IR (cm-1): 3300,2950,2220; 13C NMR: 5 signals
A)1,2-dimethylcyclopentene
B)5-methyl-2-hexyne
C)3,3-dimethyl-1-pentyne
D)4,4-dimethyl-1-pentyne
E)2,4-dimethyl-2-pentene
A)1,2-dimethylcyclopentene
B)5-methyl-2-hexyne
C)3,3-dimethyl-1-pentyne
D)4,4-dimethyl-1-pentyne
E)2,4-dimethyl-2-pentene

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
75
Give one reason why 13C NMR is less sensitive than 1H NMR.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
76
Why is carbon-hydrogen coupling not generally seen in 1H NMR spectra?

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
77
Give the structure of a compound that has a formula of C6H12O and has a triplet (3H,1.1 ppm),doublet (6H,1.2 ppm),quartet (2H,2.4 ppm),and septet (1H,2.7 ppm)in the 1H NMR spectrum.
A)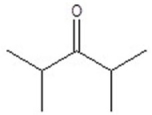
B)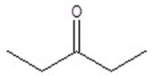
C)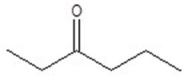
D)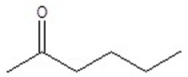
E)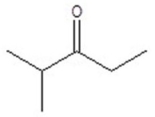
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
78
Deduce the identity of the compound from the data provided. C8H13Br: IR (cm-1): 2950,2150
1H NMR (δ,splitting,integral): 3.5 (t,2H),1.8 (t,2H),
0)9 (s,9H)
13C NMR: 6 signals
A)CH3CHBrCCC(CH3)3
B)HCCCH2C(CH3)2CH2CH2Br
C)3-bromo-1,2-dimethylcyclohexene
D)4-bromo-1,2,4-trimethylcyclopentene
E)BrCH2CH2CCC(CH3)3
1H NMR (δ,splitting,integral): 3.5 (t,2H),1.8 (t,2H),
0)9 (s,9H)
13C NMR: 6 signals
A)CH3CHBrCCC(CH3)3
B)HCCCH2C(CH3)2CH2CH2Br
C)3-bromo-1,2-dimethylcyclohexene
D)4-bromo-1,2,4-trimethylcyclopentene
E)BrCH2CH2CCC(CH3)3

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
79
Deduce the identity of the compound from the data provided.
C5H10O2: IR (cm-1): 2950,1740; 13C NMR (δ,splitting): 15.8 (q),19.7 (q),68.4 (d),195.3 (s)
C5H10O2: IR (cm-1): 2950,1740; 13C NMR (δ,splitting): 15.8 (q),19.7 (q),68.4 (d),195.3 (s)

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
80
How many distinct carbon signals are expected in the proton-decoupled 13C NMR spectrum of the compound below? 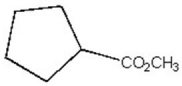


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck


