Exam 14: Nmr Spectroscopy
Exam 1: Remembering General Chemistry: Electronic Structure and Bonding90 Questions
Exam 2: Acids and Bases: Central to Understanding Organic Chemistry43 Questions
Exam 3: An Introduction to Organic Compounds: Nomenclature, physical Properties, and Structure136 Questions
Exam 4: Isomers: the Arrangement of Atoms in Space125 Questions
Exam 5: Alkenes: Structure,nomenclature,and an Introduction to Reactivity - Thermodynamics and Kinetics84 Questions
Exam 6: The Reactions of Alkenes - the Stereochemistry of Addition Reactions89 Questions
Exam 7: The Reactions of Alkynes - Introduction to Multistep Synthesis124 Questions
Exam 8: Delocalized Electrons: Their Effect on Stability, pka, and the Products of a Reaction - Aromaticity and Electronic Effects: an Introduction to the Reactions of Benzene185 Questions
Exam 9: Substitution and Elimination Reactions of Alkyl Halides228 Questions
Exam 10: Reactions of Alcohols, ethers, epoxides, amines and Sulfur-Containing Compounds109 Questions
Exam 11: Organometallic Compounds65 Questions
Exam 12: Radicals141 Questions
Exam 13: Mass Spectrometry,infrared Spectroscopy,and Uvvis Spectroscopy140 Questions
Exam 14: Nmr Spectroscopy122 Questions
Exam 15: Reactions of Carboxylic Acids and Carboxylic Acid Derivatives126 Questions
Exam 16: Reactions of Aldehydes and Ketones122 Questions
Exam 17: Reactions at the Α-Carbon121 Questions
Exam 18: Reactions of Benzene and Substituted Benzenes168 Questions
Exam 19: More About Amines - Reactions of Heterocylic Compounds126 Questions
Exam 20: The Organic Chemistry of Carbohydrates110 Questions
Exam 21: Amino Acids,peptides,and Proteins117 Questions
Exam 22: Catalysis in Organic Reactions and in Enzymatic Reactions92 Questions
Exam 23: The Organic Chemistry of the Coenzymes, compounds Derived From Vitamins102 Questions
Exam 24: The Organic Chemistry of the Metabolic Pathways90 Questions
Exam 25: The Organic Chemistry of Lipids37 Questions
Exam 26: The Chemistry of the Nucleic Acids94 Questions
Exam 27: Synthetic Polymers116 Questions
Exam 28: Pericyclic Reactions102 Questions
Select questions type
Deduce the identity of the compound from the data provided. C7H12: IR (cm-1): 3300,2950,2220; 13C NMR: 5 signals
Free
(Multiple Choice)
5.0/5  (41)
(41)
Correct Answer:
D
Deduce the identity of the compound from the data provided. C5H8O4: IR (cm-1): 2800-3300 (broad),2950,1740
13C NMR (δ,splitting): 17.3 (q),44.3 (s),210.5 (s)
Free
(Multiple Choice)
4.8/5  (34)
(34)
Correct Answer:
A
How many signals would you expect to see in the 1H NMR spectrum of the following compound? 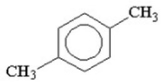
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
E
Deduce the identity of the following compound from the spectral data given.
C9H10O2: 13C NMR,δ 18.06 (quartet),45.40 (doublet),127.32 (doublet),127.55 (doublet),128.61 (doublet),139.70 (singlet),180.98 (singlet); IR,broad 3500-2800,1708 cm-1
(Short Answer)
4.9/5  (31)
(31)
Give the structure of a compound that has a formula of C9H10O2 and has signals in the 13C NMR spectrum at 13.6 ppm (CH3); 59.1 ppm (CH2); 128.4 ppm (CH); 129.7 ppm (CH); 130.5 ppm (C); 132.8 ppm (CH); and 167.0 ppm (C).
(Multiple Choice)
4.8/5  (32)
(32)
Deduce the identity of the following compound from the 1H NMR data given.
C8H10O: δ 3.4 (3H,singlet),4.5 (2H,singlet),7.2 (5H,singlet)
(Short Answer)
4.9/5  (34)
(34)
Give the splitting pattern for each proton in the 1H NMR spectrum. 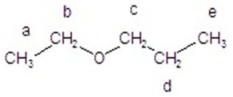
(Multiple Choice)
4.9/5  (33)
(33)
How many signals will be observable in the DEPT 13C NMR spectrum of naphthalene (shown below)? 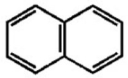
(Multiple Choice)
4.9/5  (26)
(26)
Deduce the identity of the following compound from the 1H NMR spectral data given.
C8H18O : δ 0.89 (6H,doublet),1.87 (1H,multiplet),3.17 (2H,doublet)(ppm)
(Essay)
4.7/5  (23)
(23)
________ is commonly used as an internal reference in NMR spectroscopy; its signal is assigned δ=0 in 1H and 13C NMR spectroscopy.
(Essay)
4.7/5  (29)
(29)
Deduce the identity of the compound from the data provided. C8H13Br: IR (cm-1): 2950,2150
1H NMR (δ,splitting,integral): 3.5 (t,2H),1.8 (t,2H),
0)9 (s,9H)
13C NMR: 6 signals
(Multiple Choice)
4.8/5  (23)
(23)
Why is carbon-hydrogen coupling not generally seen in 1H NMR spectra?
(Essay)
4.9/5  (28)
(28)
The chair form of cyclohexane has protons in two distinct environments,axial and equatorial.When the proton NMR of cyclohexane is run on a 100 MHz instrument at 23°C,only one signal for the compound is observed.Explain this apparent contradiction.
(Essay)
4.8/5  (38)
(38)
Which of the following protons gives an NMR signal with the lowest chemical shift value (farthest upfield)? F-CH2CH2CH2CH2CH2-Br
1 2 3 4 5
(Multiple Choice)
4.7/5  (36)
(36)
Deduce the structure from the data given: C8H10O; 13C NMR δ (coupling): 140 (s),128 (d),126 (d),122 (d),60 (t),15 (q).
(Short Answer)
4.7/5  (33)
(33)
Which of the following electromagnetic wave types is used in nuclear magnetic resonance spectroscopy?
(Multiple Choice)
4.9/5  (26)
(26)
Provide the structure that is consistent with the data below.
C7H14O2
IR (cm-1): 2950,1750
1H NMR (d): 2.3 (2H,q),1.0 (3H,t),0.9 (9H,s)
13C NMR (d): 185 (s),78 (s),29 (t),14 (q),12 (q)
(Essay)
4.8/5  (30)
(30)
What splitting pattern is observed in the proton NMR spectrum for the indicated hydrogens? 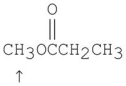
(Multiple Choice)
4.8/5  (41)
(41)
Deduce the identity of the following compound from the spectral data given.
C5H10O: 1H NMR,δ 1.2 (6H,doublet),2.1 (3H,singlet),2.8 (1H,septet); IR,2980,1710 cm-1; MS,m/z 71,
(Essay)
4.7/5  (31)
(31)
Give the structure of a compound that has a formula of C5H10O2, and has a triplet (3H,1.1 ppm),sextet (2H,1.8 ppm),triplet (2H,2.3 ppm)and a singlet (3H,3.8 ppm)in the 1H NMR spectrum.
(Multiple Choice)
4.9/5  (37)
(37)
Showing 1 - 20 of 122
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)