Deck 17: Reactions at the Α-Carbon
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/121
Play
Full screen (f)
Deck 17: Reactions at the Α-Carbon
1
Which of the following compounds has the most stable enol tautomer?
A)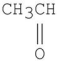
B)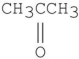
C)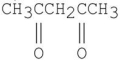
D)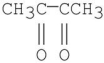
E)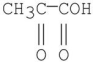
A)

B)
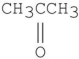
C)
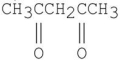
D)
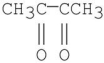
E)
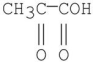
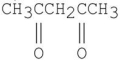
2
Which of the following is a β-diester?
A)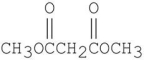
B)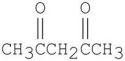
C)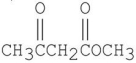
D)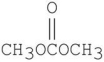
E)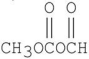
A)
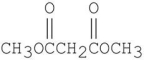
B)
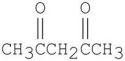
C)
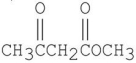
D)
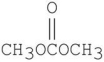
E)
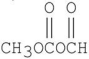
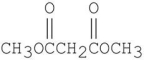
3
Which of the following underlined alpha hydrogens are the most acidic?
A)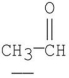
B)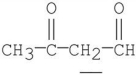
C)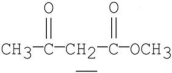
D)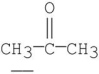
E)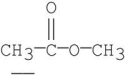
A)

B)
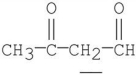
C)

D)

E)
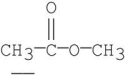
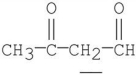
4
Which of the labeled hydrogen atoms in the following structure is the most acidic? 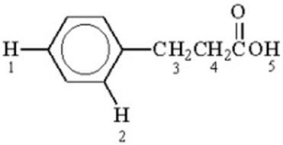
A)1
B)2
C)3
D)4
E)5

A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
5
Draw the most stable enol tautomer of the ketone shown below. 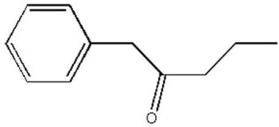


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
6
Use an asterisk to indicate the most electron-rich carbon in the molecule below. 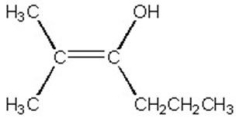


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
7
Which compound has the lowest pKa?
A)(CH3CH2O2C)2CH2
B)CH3CH2O2CCH2CHO
C)CH3CH2COCH2CHO
D)PhCOCH2COCH3
E)(NC)2CH2
A)(CH3CH2O2C)2CH2
B)CH3CH2O2CCH2CHO
C)CH3CH2COCH2CHO
D)PhCOCH2COCH3
E)(NC)2CH2

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the labeled hydrogen atoms in the following structure is the most acidic? 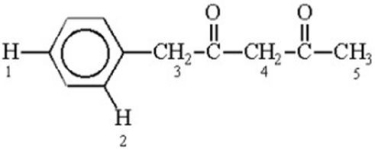
A)1
B)2
C)3
D)4
E)5

A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
9
Provide a detailed,stepwise mechanism for the base-catalyzed enolization of acetaldehyde.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following keto-enol tautomers is more stable? Explain. 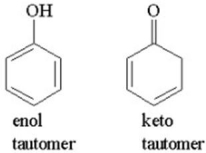


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
11
Rank the following compounds in order of increasing acidity of their a-Hs,least acidic to most acidic: CH3CH2CON(CH3)2,PhCOCH2CHO,CH3CH2CO2CH2CH3,and PhCOCH2CO2CH2CH3.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
12
When compared to the keto form,the enol form of which of the following compounds is most stable? 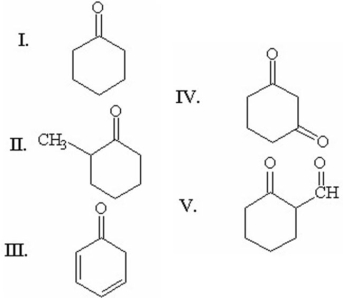
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following compounds does not have an alpha hydrogen? 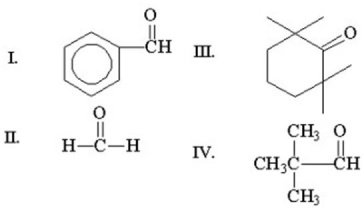
A)I
B)II
C)III
D)IV
E)all of the above

A)I
B)II
C)III
D)IV
E)all of the above

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is the most stable enol form of 1,3-cyclohexanedione? 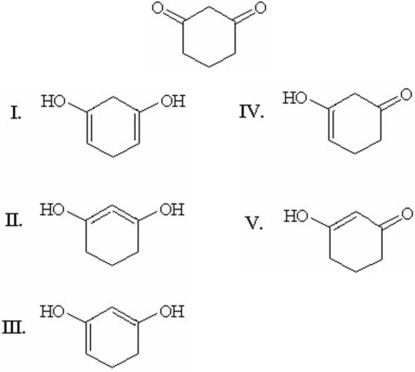
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
15
Which compound's α H is the least acidic?
A)nitroethane
B)propanenitrile
C)N,N-dimethylacetamide
D)propanal
E)acetone
A)nitroethane
B)propanenitrile
C)N,N-dimethylacetamide
D)propanal
E)acetone

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
16
Why is the pKa of acetone 20 while that of ethane is 50? Explain the origin for this difference in acidity.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
17
List the following carbonyl compounds in order of decreasing pKa: 2,4-pentanedione,diethyl malonate,propanal,ethyl acetate.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
18
How many Hs in the compound below are replaced by Ds when it is shaken in D2O containing trace hydroxide? 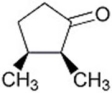
A)1
B)2
C)3
D)6
E)7

A)1
B)2
C)3
D)6
E)7

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the labeled hydrogen atoms in the following structure is the most acidic? 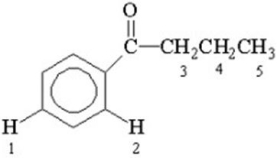
A)1
B)2
C)3
D)4
E)5

A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
20
Provide a detailed,stepwise mechanism for the acid-catalyzed enolization of acetaldehyde.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
21
Provide the major organic product of the following. 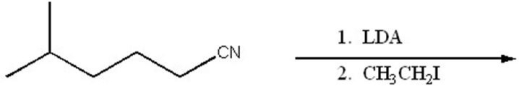


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
22
Give the major product for the following reaction. 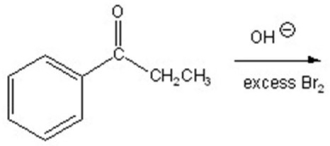
A)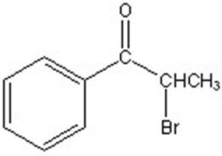
B)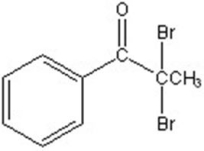
C)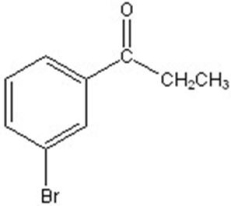
D)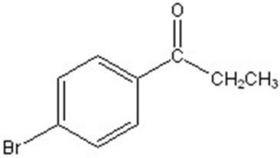
E)no reaction

A)

B)

C)

D)

E)no reaction

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
23
What iminium salt is produced in the reaction shown below? 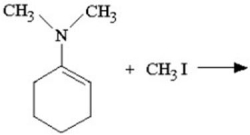


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
24
Provide the major organic product of the following. 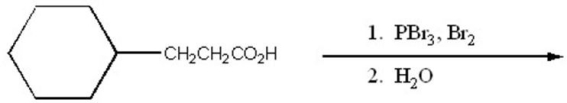


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
25
Provide a detailed,stepwise mechanism for the formation of acetate and bromodiiodomethane from bromoacetone,hydroxide and iodine.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
26
Provide the major organic product of the following. 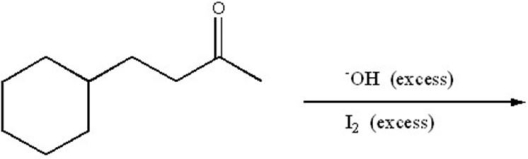


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
27
What is the carbon nucleophile which attacks molecular bromine in the acid-catalyzed α-bromination of a ketone?
A)an enolate
B)a Grignard reagent
C)an acetylide
D)a carbocation
E)an enol
A)an enolate
B)a Grignard reagent
C)an acetylide
D)a carbocation
E)an enol

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
28
Give the major product for the following reaction. 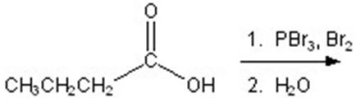
A)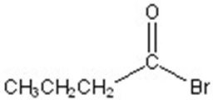
B)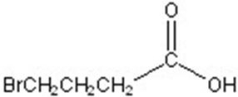
C)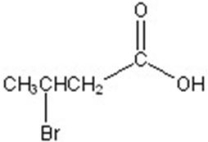
D)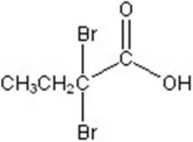
E)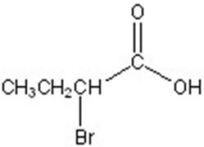

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
29
Which is the major organic product from the following reaction? 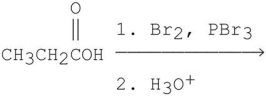
A)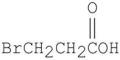
B)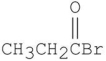
C)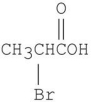
D)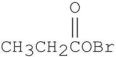
E)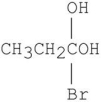

A)
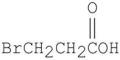
B)
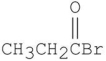
C)

D)
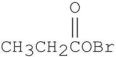
E)


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following methods can be used to prepare the following compound? 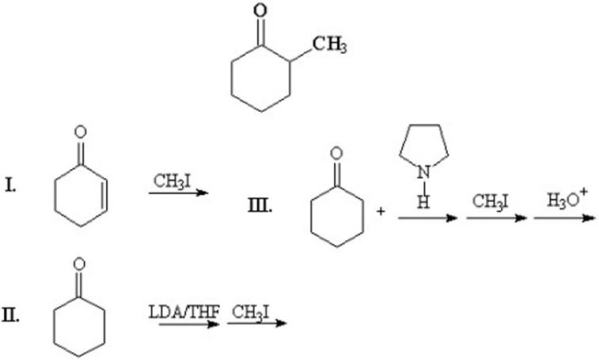
A)I
B)II
C)III
D)II and III
E)I,II,and III

A)I
B)II
C)III
D)II and III
E)I,II,and III

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following will alkylate a lithium enolate most rapidly?
A)methyl bromide
B)isopropyl bromide
C)neopentyl bromide
D)bromobenzene
E)2-methylbromobenzene
A)methyl bromide
B)isopropyl bromide
C)neopentyl bromide
D)bromobenzene
E)2-methylbromobenzene

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
32
The Hell-Volhard-Zelinsky reaction involves
A)the α-bromination of carboxylic acids.
B)the α-bromination of ketones.
C)the bromination of alcohols.
D)the oxidation of aldehydes to acids.
E)none of the above.
A)the α-bromination of carboxylic acids.
B)the α-bromination of ketones.
C)the bromination of alcohols.
D)the oxidation of aldehydes to acids.
E)none of the above.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following reagents will quantitatively convert an enolizable ketone to its enolate salt?
A)lithium hydroxide
B)lithium diisopropylamide
C)methyllithium
D)diethylamine
E)pyridine
A)lithium hydroxide
B)lithium diisopropylamide
C)methyllithium
D)diethylamine
E)pyridine

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
34
Give the major product for the following reaction. 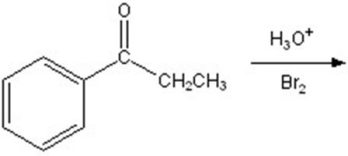
A)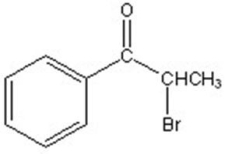
B)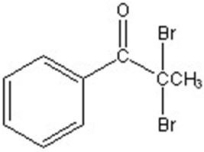
C)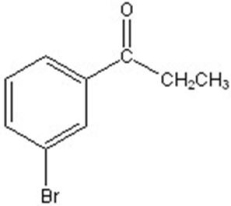
D)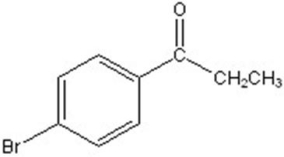
E)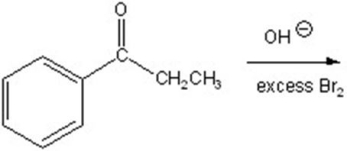

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
35
A student proposed the following synthesis.Why did it fail? 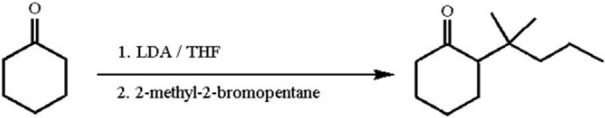
A)The use of LDA results in the thermodynamic product instead of the desired kinetic product.
B)LDA is insoluble in the THF solvent so the reaction is too slow to occur at a useful rate.
C)LDA acts as a nucleophile instead of a base in its reaction with cyclohexanone.
D)The tertiary bromide is too sterically hindered to be attacked by the enolate.
E)Instead of 2-methyl-2-bromopentane,2-bromo-3-methylpentane should have been used to anticipate a cationic rearrangement that would occur.

A)The use of LDA results in the thermodynamic product instead of the desired kinetic product.
B)LDA is insoluble in the THF solvent so the reaction is too slow to occur at a useful rate.
C)LDA acts as a nucleophile instead of a base in its reaction with cyclohexanone.
D)The tertiary bromide is too sterically hindered to be attacked by the enolate.
E)Instead of 2-methyl-2-bromopentane,2-bromo-3-methylpentane should have been used to anticipate a cationic rearrangement that would occur.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
36
How would you accomplish the following conversion? 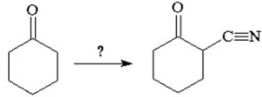
A)H+/H2O; HC N
N
B)(-OH/NaC N)
N)
C)Br2/H+,H2O; NaC N
N
D)HBr; NaC N
N
E)LiAlH4; HC N; KMnO4
N; KMnO4

A)H+/H2O; HC
 N
NB)(-OH/NaC
 N)
N)C)Br2/H+,H2O; NaC
 N
ND)HBr; NaC
 N
NE)LiAlH4; HC
 N; KMnO4
N; KMnO4
Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
37
What is the major organic product of the following reaction? 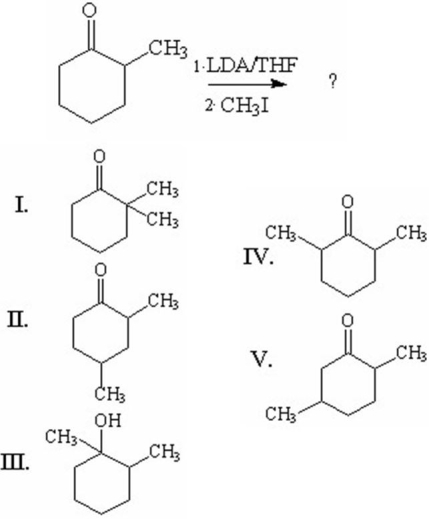
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following compounds will give a positive iodoform test?
A)propanal
B)2-pentanone
C)3-pentanone
D)benzophenone
E)cyclohexanone
A)propanal
B)2-pentanone
C)3-pentanone
D)benzophenone
E)cyclohexanone

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
39
Provide a detailed,stepwise mechanism for the α-bromination of acetone in base.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
40
Enolates are frequently formed by treating an aldehyde or ketone with LDA.However,the LDA must first be generated by reacting diisopropylamine with butyllithium.Why don't chemists just use butyllithium itself to deprotonate the aldehyde or ketone and form the enolate?

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
41
In the Michael reaction,addition to the α,β-unsaturated carbonyl occurs in a
A)1,2-fashion
B)1,3-fashion
C)1,4-fashion
D)1,5-fashion
E)Diels-Alder reaction
A)1,2-fashion
B)1,3-fashion
C)1,4-fashion
D)1,5-fashion
E)Diels-Alder reaction

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
42
Give the major product for the following reaction. 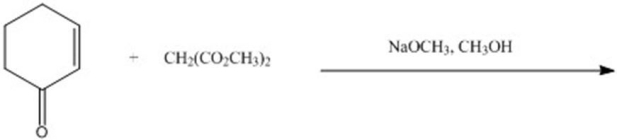


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
43
An enolate attacks an aldehyde and the resulting product is subsequently protonated.What type of reaction is this?
A)a Fischer esterification
B)an acid-catalyzed aldol condensation
C)a base-mediated aldol condensation
D)a Hell-Volhard-Zelinsky reaction
E)a Selman-Jones reaction
A)a Fischer esterification
B)an acid-catalyzed aldol condensation
C)a base-mediated aldol condensation
D)a Hell-Volhard-Zelinsky reaction
E)a Selman-Jones reaction

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
44
Show how an enolate can add to a carbonyl.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
45
Give the major product for the reaction. 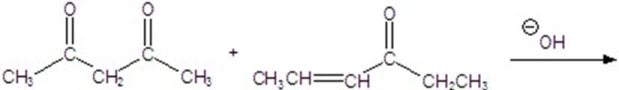
A)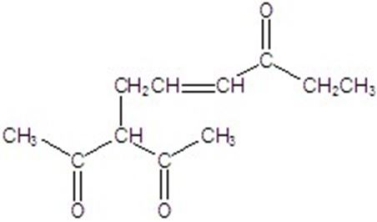
B)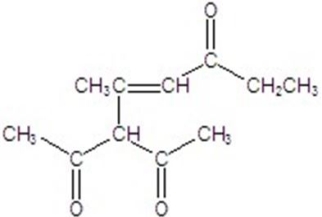
C)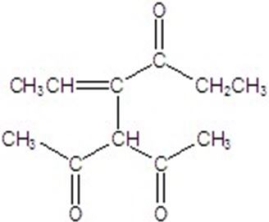
D)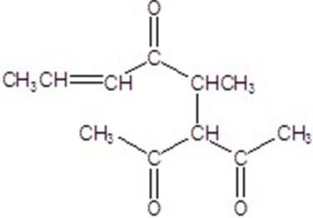
E)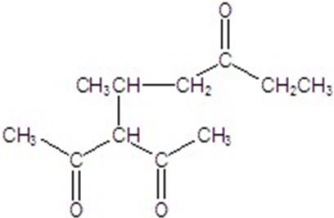
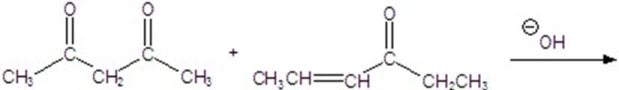
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
46
What is the major organic product of the following reaction? 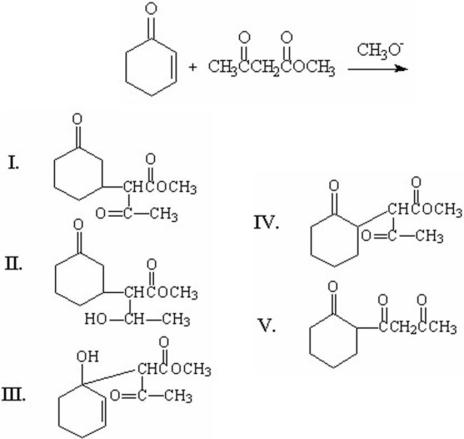
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
47
Provide the major organic product(s)of the reaction shown below. 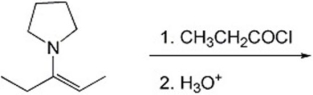


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
48
What is the major organic product of the following aldol addition? 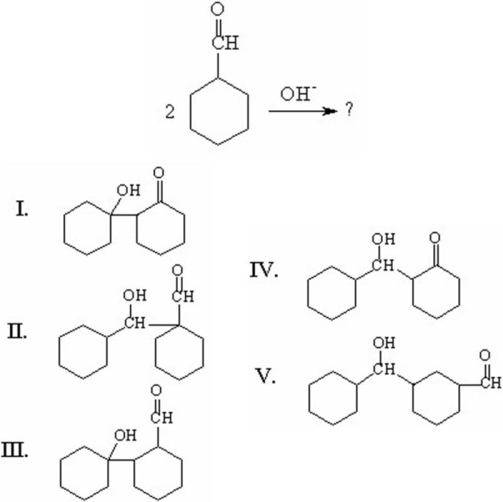
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
49
What is the major organic product from the following reaction? 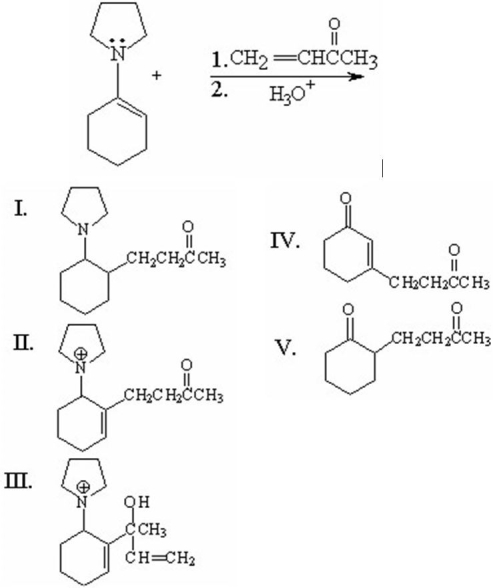
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
50
Give the major product for the following reaction. 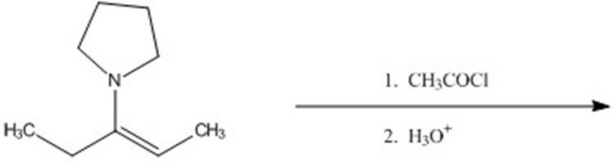


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
51
What is the molecular formula of the major organic product of the following reaction sequence? 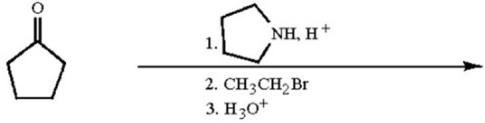
A)C7H12O
B)C7H14O
C)C11H19N
D)C11H21N
E)C11H23N

A)C7H12O
B)C7H14O
C)C11H19N
D)C11H21N
E)C11H23N

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following statements describes the first step in the mechanism of the aldol condensation?
A)An alpha hydrogen is abstracted by the base to form an enolate anion.
B)A nucleophilic base attacks the carbonyl carbon atom.
C)The carbonyl oxygen is protonated by the base ion.
D)The alpha hydrogen is abstracted by an acid to the enolate anion.
E)The carbonyl oxygen of one aldehyde attacks the carbonyl carbon of another.
A)An alpha hydrogen is abstracted by the base to form an enolate anion.
B)A nucleophilic base attacks the carbonyl carbon atom.
C)The carbonyl oxygen is protonated by the base ion.
D)The alpha hydrogen is abstracted by an acid to the enolate anion.
E)The carbonyl oxygen of one aldehyde attacks the carbonyl carbon of another.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
53
Give the major product for the following reaction. 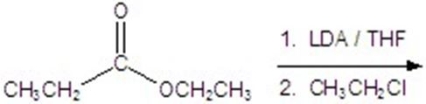
A)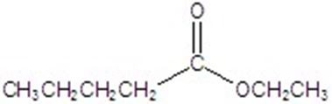
B)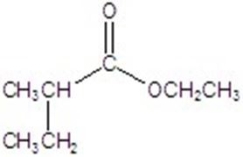
C)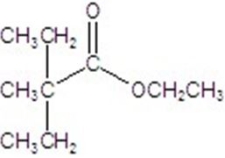
D)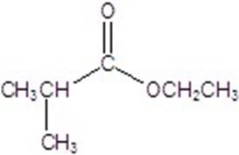
E)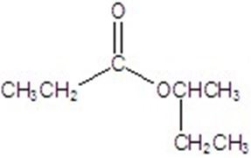
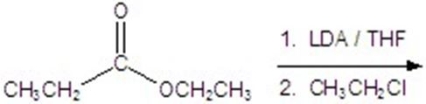
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
54
Give the major product for the following reaction. 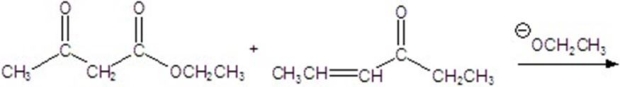


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
55
Give the major product for the following reaction. 
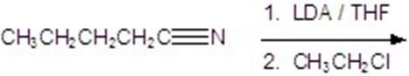

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
56
Give the major product for the following reaction. 


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
57
Give the major product for the following reaction. 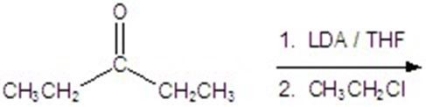
A)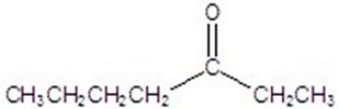
B)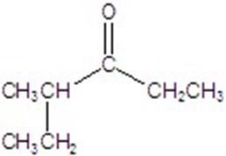
C)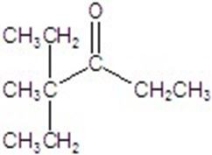
D)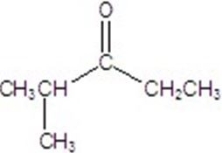
E)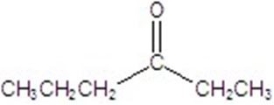
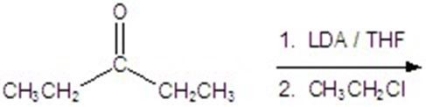
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
58
Provide the major organic product(s)of the reaction shown below. 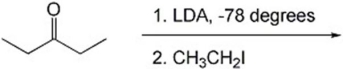


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following aldehydes does not undergo an aldol addition when mixed with base? 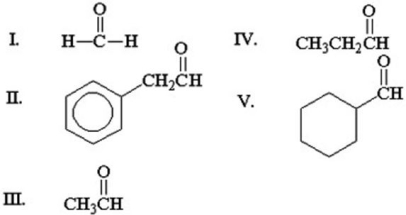
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
60
Give the major product for the reaction. 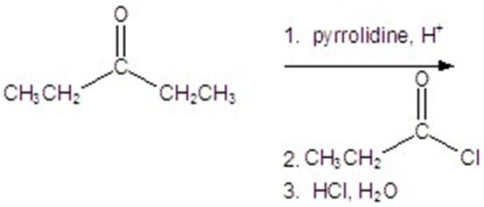
A)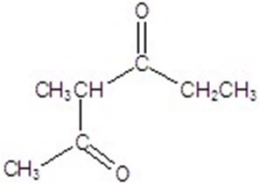
B)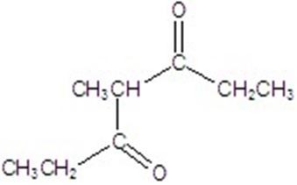
C)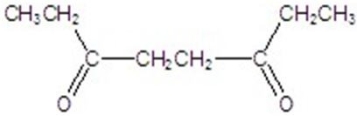
D)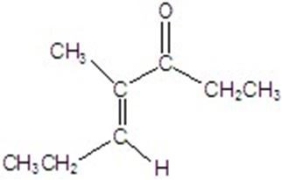
E)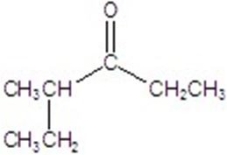

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
61
Provide the structure of the Claisen product in the self-condensation of methyl phenylacetate.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
62
What materials are needed to prepare the following product? 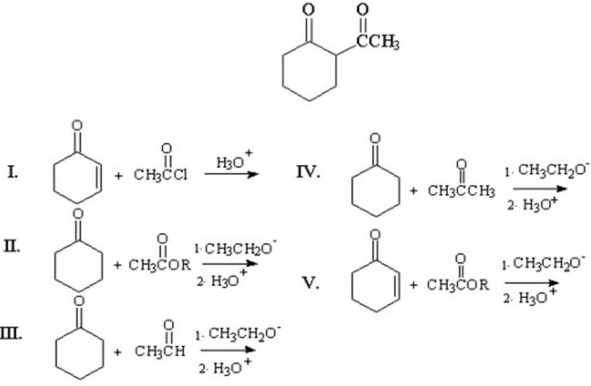
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
63
What is the major product of the following condensation? 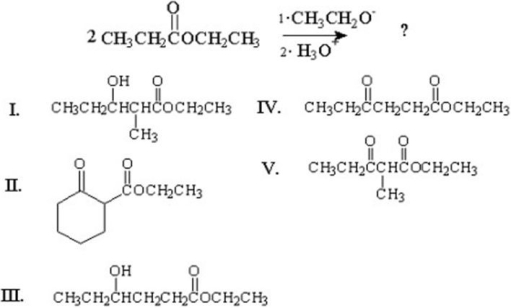
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
64
Give the major product for the following reaction. 


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
65
What two molecules were condensed in an aldol reaction to produce PhCH  CHCOPh?
CHCOPh?
 CHCOPh?
CHCOPh?
Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
66
What two starting materials yield OHCCH2CO2CH2CH3 as the mixed Claisen condensation product?

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
67
What two molecules were condensed in an aldol reaction to produce
(CH3)3CCH CHCOCH3?
CHCOCH3?
(CH3)3CCH
 CHCOCH3?
CHCOCH3?
Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
68
Show how the compound below could be prepared by a synthetic route that uses a Claisen condensation. 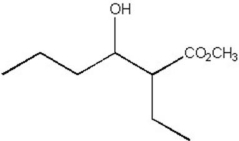


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
69
Provide the major organic product(s)of the reaction shown below. 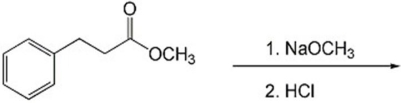


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following could result from the dehydration of an aldol?
A)4-methyl-3-penten-2-one
B)4-methyl-4-penten-2-one
C)4-methyl-5-hexen-2-one
D)4-methyl-4-hexen-2-one
E)3-methyl-4-penten-2-one
A)4-methyl-3-penten-2-one
B)4-methyl-4-penten-2-one
C)4-methyl-5-hexen-2-one
D)4-methyl-4-hexen-2-one
E)3-methyl-4-penten-2-one

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
71
Provide a synthesis of 2-ethylhexanol using butanal as the only source of carbon.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
72
Give the major product of the following reaction. 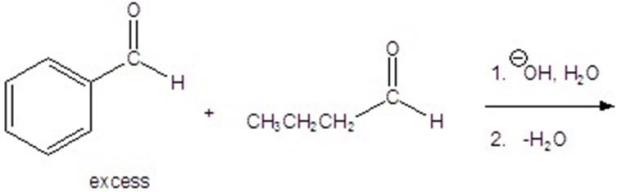


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
73
What is the major organic product of the following addition? 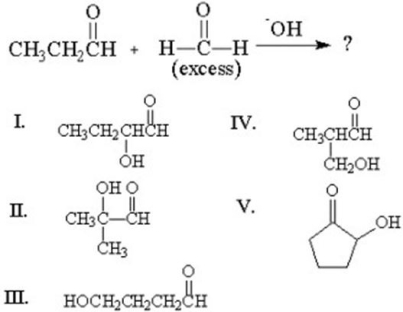
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
74
Give the major product for the following reaction. 


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
75
Provide the structure of the major organic product that results when 4-methylhexanal is heated in base and undergoes and aldol addition followed by a dehydration.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
76
(a)The base-catalyzed reaction of 1 mole of acetone with 2 moles of benzaldehyde yields a yellow solid,C17H14O.What is the structure of the product?
(b)What will be the result when only 1 mole of benzaldehyde is added?
(c)What will be the result when acetone is not added at all?
(d)What will be the result when benzaldehyde is not added at all?
(e)Why is the self-condensation of acetone unlikely in the presence of benzaldehyde?
(b)What will be the result when only 1 mole of benzaldehyde is added?
(c)What will be the result when acetone is not added at all?
(d)What will be the result when benzaldehyde is not added at all?
(e)Why is the self-condensation of acetone unlikely in the presence of benzaldehyde?

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
77
Propose a stepwise mechanism that would explain the following conversion. 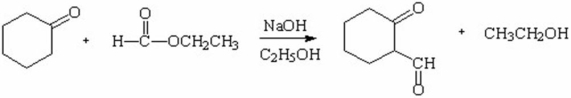


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
78
Give the major product of the following reaction. 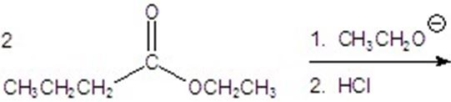
A)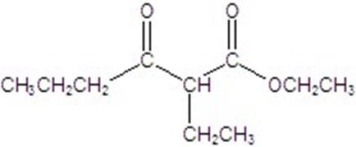
B)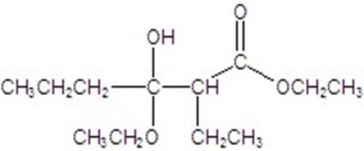
C)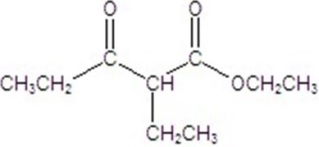
D)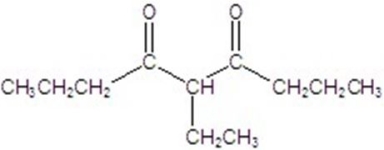
E)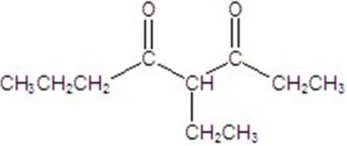

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
79
Give the major product for the following reaction. 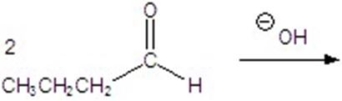
A)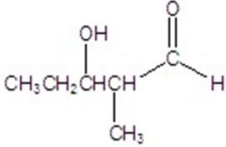
B)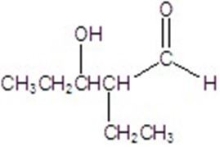
C)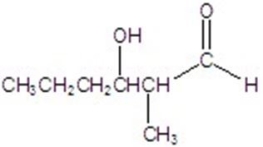
D)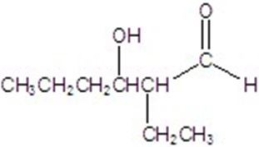
E)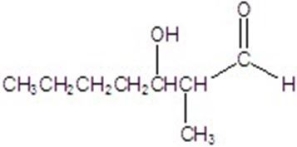

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
80
In theory a poorly planned crossed aldol reaction can produce how many different aldol regioisomers?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck


