Deck 3: Stoichiometry of Formulas and Equations
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/90
Play
Full screen (f)
Deck 3: Stoichiometry of Formulas and Equations
1
Lead(II)nitrate is a poisonous substance which has been used in the manufacture of special explosives and as a sensitizer in photography.Calculate the mass of lead in 139 g of Pb(NO3)2.
A)107 g
B)90.8 g
C)87.0 g
D)83.4 g
E)62.6 g
A)107 g
B)90.8 g
C)87.0 g
D)83.4 g
E)62.6 g
87.0 g
2
Calculate the number of moles in 17.8 g of the antacid magnesium hydroxide,Mg(OH)2.
A)3.28 mol
B)2.32 mol
C)0.431 mol
D)0.305 mol
E)0.200 mol
A)3.28 mol
B)2.32 mol
C)0.431 mol
D)0.305 mol
E)0.200 mol
0.305 mol
3
Sodium bromate is used in a mixture which dissolves gold from its ores.Calculate the mass in grams of 4.68 mol of sodium bromate.
A)706 g
B)482 g
C)383 g
D)32.2 g
E)0.0310 g
A)706 g
B)482 g
C)383 g
D)32.2 g
E)0.0310 g
706 g
4
Calculate the molar mass of Ca(BO2)2·6H2O.
A)273.87 g/mol
B)233.79 g/mol
C)183.79 g/mol
D)174.89 g/mol
E)143.71 g/mol
A)273.87 g/mol
B)233.79 g/mol
C)183.79 g/mol
D)174.89 g/mol
E)143.71 g/mol

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
5
Calculate the molar mass of tetraphosphorus decaoxide,P4O10,a corrosive substance which can be used as a drying agent.
A)469.73 g/mol
B)283.89 g/mol
C)190.97 g/mol
D)139.88 g/mol
E)94.97 g/mol
A)469.73 g/mol
B)283.89 g/mol
C)190.97 g/mol
D)139.88 g/mol
E)94.97 g/mol

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
6
Aluminum sulfate,Al2(SO4)3,is used in tanning leather,purifying water,and manufacture of antiperspirants.Calculate its molar mass.
A)450.06 g/mol
B)342.15 g/mol
C)315.15 g/mol
D)278.02 g/mol
E)74.98 g/mol
A)450.06 g/mol
B)342.15 g/mol
C)315.15 g/mol
D)278.02 g/mol
E)74.98 g/mol

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
7
The number of hydrogen atoms in 0.050 mol of C3H8O3 is
A)3.0 1022 H atoms
B)1.2 1023 H atoms
C)2.4 1023 H atoms
D)4.8 1023 H atoms
E)none of these choices is correct
A)3.0 1022 H atoms
B)1.2 1023 H atoms
C)2.4 1023 H atoms
D)4.8 1023 H atoms
E)none of these choices is correct

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
8
Calcium fluoride,CaF2,is a source of fluorine and is used to fluoridate drinking water.Calculate its molar mass.
A)118.15 g/mol
B)99.15 g/mol
C)78.07 g/mol
D)59.08 g/mol
E)50.01 g/mol
A)118.15 g/mol
B)99.15 g/mol
C)78.07 g/mol
D)59.08 g/mol
E)50.01 g/mol

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
9
What is the mass in grams of 0.250 mol of the common antacid calcium carbonate?
A)4.00 102 g
B)25.0 g
C)17.0 g
D)4.00 10-2 g
E)2.50 10-3 g
A)4.00 102 g
B)25.0 g
C)17.0 g
D)4.00 10-2 g
E)2.50 10-3 g

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
10
Aluminum oxide,Al2O3,is used as a filler for paints and varnishes as well as in the manufacture of electrical insulators.Calculate the number of moles in 47.51 g of Al2O3.
A)2.377 mol
B)2.146 mol
C)1.105 mol
D)0.4660 mol
E)0.4207 mol
A)2.377 mol
B)2.146 mol
C)1.105 mol
D)0.4660 mol
E)0.4207 mol

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
11
Calculate the mass in grams of 8.35 1022 molecules of CBr4.
A)0.0217 g
B)0.139 g
C)7.21 g
D)12.7 g
E)46.0 g
A)0.0217 g
B)0.139 g
C)7.21 g
D)12.7 g
E)46.0 g

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
12
Sulfur trioxide can react with atmospheric water vapor to form sulfuric acid that falls as acid rain.Calculate the mass in grams of 3.65 1020 molecules of SO3.
A)6.06 10-4 g
B)2.91 10-2 g
C)4.85 10-2 g
D)20.6 g
E)1650 g
A)6.06 10-4 g
B)2.91 10-2 g
C)4.85 10-2 g
D)20.6 g
E)1650 g

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
13
Magnesium fluoride is used in the ceramics and glass industry.What is the mass of 1.72 mol of magnesium fluoride?
A)43.3 g
B)62.3 g
C)74.5 g
D)92.9 g
E)107 g
A)43.3 g
B)62.3 g
C)74.5 g
D)92.9 g
E)107 g

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
14
Potassium dichromate,K2Cr2O7,is used in tanning leather,decorating porcelain and waterproofing fabrics.Calculate the number of chromium atoms in 78.82 g of K2Cr2O7.
A)9.490 1025 Cr atoms
B)2.248 1024 Cr atoms
C)1.124 1024 Cr atoms
D)3.227 1023 Cr atoms
E)1.613 1023 Cr atoms
A)9.490 1025 Cr atoms
B)2.248 1024 Cr atoms
C)1.124 1024 Cr atoms
D)3.227 1023 Cr atoms
E)1.613 1023 Cr atoms

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
15
Copper(II)sulfate pentahydrate,CuSO4·5H2O,is used as a fungicide and algicide.Calculate the mass of oxygen in 1.000 mol of CuSO4·5H2O.
A)249.7 g
B)144.0 g
C)96.00 g
D)80.00 g
E)64.00 g
A)249.7 g
B)144.0 g
C)96.00 g
D)80.00 g
E)64.00 g

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
16
Calculate the number of oxygen atoms in 29.34 g of sodium sulfate,Na2SO4.
A)1.244 1023 O atoms
B)4.976 1023 O atoms
C)2.409 1024 O atoms
D)2.915 1024 O atoms
E)1.166 1025 O atoms
A)1.244 1023 O atoms
B)4.976 1023 O atoms
C)2.409 1024 O atoms
D)2.915 1024 O atoms
E)1.166 1025 O atoms

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
17
Household sugar,sucrose,has the molecular formula C12H22O11.What is the % of carbon in sucrose,by mass?
A)26.7 %
B)33.3 %
C)41.4 %
D)42.1 %
E)52.8 %
A)26.7 %
B)33.3 %
C)41.4 %
D)42.1 %
E)52.8 %

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
18
Phosphorus pentachloride,PCl5,a white solid that has a pungent,unpleasant odor,is used as a catalyst for certain organic reactions.Calculate the number of moles in 38.7 g of PCl5.
A)5.38 mol
B)3.55 mol
C)0.583 mol
D)0.282 mol
E)0.186 mol
A)5.38 mol
B)3.55 mol
C)0.583 mol
D)0.282 mol
E)0.186 mol

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
19
Calculate the molar mass of rubidium carbonate,Rb2CO3.
A)340.43 g/mol
B)255.00 g/mol
C)230.94 g/mol
D)145.47 g/mol
E)113.48 g/mol
A)340.43 g/mol
B)255.00 g/mol
C)230.94 g/mol
D)145.47 g/mol
E)113.48 g/mol

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
20
Calculate the molar mass of (NH4)3AsO4.
A)417.80 g/mol
B)193.03 g/mol
C)165.02 g/mol
D)156.96 g/mol
E)108.96 g/mol
A)417.80 g/mol
B)193.03 g/mol
C)165.02 g/mol
D)156.96 g/mol
E)108.96 g/mol

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
21
Hydroxylamine nitrate contains 29.17 mass % N,4.20 mass % H,and 66.63 mass % O.Determine its empirical formula.
A)HNO
B)H2NO2
C)HN6O16
D)HN16O7
E)H2NO3
A)HNO
B)H2NO2
C)HN6O16
D)HN16O7
E)H2NO3

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
22
Ammonia will react with fluorine to produce dinitrogen tetrafluoride and hydrogen fluoride (used in production of aluminum,in uranium processing,and in frosting of light bulbs).
2NH3(g)+ 5F2(g) N2F4(g)+ 6HF(g)
How many moles of NH3 are needed to react completely with 13.6 mol of F2?
A)34.0 mol
B)27.2 mol
C)6.80 mol
D)5.44 mol
E)2.27 mol
2NH3(g)+ 5F2(g) N2F4(g)+ 6HF(g)
How many moles of NH3 are needed to react completely with 13.6 mol of F2?
A)34.0 mol
B)27.2 mol
C)6.80 mol
D)5.44 mol
E)2.27 mol

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
23
Ammonia,an important source of fixed nitrogen that can be metabolized by plants,is produced using the Haber process in which nitrogen and hydrogen combine.
N2(g)+ 3H2(g) 2NH3(g)
How many grams of nitrogen are needed to produce 325 grams of ammonia?
A)1070 g
B)535 g
C)267 g
D)178 g
E)108 g
N2(g)+ 3H2(g) 2NH3(g)
How many grams of nitrogen are needed to produce 325 grams of ammonia?
A)1070 g
B)535 g
C)267 g
D)178 g
E)108 g

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
24
Sulfur dioxide reacts with chlorine to produce thionyl chloride (used as a drying agent for inorganic halides)and dichlorine oxide (used as a bleach for wood,pulp and textiles).
SO2(g)+ 2Cl2(g) SOCl2(g)+ Cl2O(g)
If 0.400 mol of Cl2 reacts with excess SO2,how many moles of Cl2O are formed?
A)0.800 mol
B)0.400 mol
C)0.200 mol
D)0.100 mol
E)0.0500 mol
SO2(g)+ 2Cl2(g) SOCl2(g)+ Cl2O(g)
If 0.400 mol of Cl2 reacts with excess SO2,how many moles of Cl2O are formed?
A)0.800 mol
B)0.400 mol
C)0.200 mol
D)0.100 mol
E)0.0500 mol

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
25
A compound containing chromium and silicon contains 73.52 mass percent chromium.Determine its empirical formula.
A)CrSi3
B)Cr2Si3
C)Cr3Si
D)Cr3Si2
E)Cr2S
A)CrSi3
B)Cr2Si3
C)Cr3Si
D)Cr3Si2
E)Cr2S

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
26
Terephthalic acid,used in the production of polyester fibers and films,is composed of carbon,hydrogen,and oxygen.When 0.6943 g of terephthalic acid was subjected to combustion analysis it produced 1.471 g CO2 and 0.226 g H2O.What is its empirical formula?
A)C2H3O4
B)C3H4O2
C)C4H3O2
D)C5H12O4
E)C2H2O
A)C2H3O4
B)C3H4O2
C)C4H3O2
D)C5H12O4
E)C2H2O

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
27
A compound of bromine and fluorine is used to make UF6,which is an important chemical in processing and reprocessing of nuclear fuel.The compound contains 58.37 mass percent bromine.Determine its empirical formula.
A)BrF
B)BrF2
C)Br2F3
D)Br3F
E)BrF3
A)BrF
B)BrF2
C)Br2F3
D)Br3F
E)BrF3

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
28
Balance the following equation:
UO2(s)+ HF(l) UF4(s)+ H2O(l)
A)UO2(s)+ 2HF(l) UF4(s)+ H2O(l)
B)UO2(s)+ 4HF(l) UF4(s)+ 2H2O(l)
C)UO2 (s)+ H4F4(l) UF4 (s)+ H4O2(l)
D)UO2(s)+ 4HF(l) UF4(s)+ 4H2O(l)
E)UO2(s)+ 8HF(l) 2UF4(s)+ 4H2O(l)
UO2(s)+ HF(l) UF4(s)+ H2O(l)
A)UO2(s)+ 2HF(l) UF4(s)+ H2O(l)
B)UO2(s)+ 4HF(l) UF4(s)+ 2H2O(l)
C)UO2 (s)+ H4F4(l) UF4 (s)+ H4O2(l)
D)UO2(s)+ 4HF(l) UF4(s)+ 4H2O(l)
E)UO2(s)+ 8HF(l) 2UF4(s)+ 4H2O(l)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
29
Aluminum will react with bromine to form aluminum bromide (used as an acid catalyst in organic synthesis).
Al(s)+ Br2(l) Al2Br6(s)[unbalanced]
How many moles of Al are needed to form 2.43 mol of Al2Br6?
A)7.29 mol
B)4.86 mol
C)2.43 mol
D)1.62 mol
E)1.22 mol
Al(s)+ Br2(l) Al2Br6(s)[unbalanced]
How many moles of Al are needed to form 2.43 mol of Al2Br6?
A)7.29 mol
B)4.86 mol
C)2.43 mol
D)1.62 mol
E)1.22 mol

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
30
Hydroxylamine hydrochloride is a powerful reducing agent which is used as a polymerization catalyst.It contains 5.80 mass % H,20.16 mass % N,23.02 mass % O,and 51.02 mass % Cl.What is its empirical formula?
A)H2N7O8Cl18
B)H2N2O2Cl
C)HN3O4Cl9
D)H4NOCl
E)H4NOCl2
A)H2N7O8Cl18
B)H2N2O2Cl
C)HN3O4Cl9
D)H4NOCl
E)H4NOCl2

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
31
Balance the following equation:
B2O3(s)+ HF(l) BF3(g)+ H2O(l)
A)B2O3(s)+ 6HF(l) 2BF3(g)+ 3H2O(l)
B)B2O3(s)+ H6F6(l) B2F6(g)+ H6O3(l)
C)B2O3(s)+ 2HF(l) 2BF3(g)+ H2O(l)
D)B2O3(s)+ 3HF(l) 2BF3(g)+ 3H2O(l)
E)B2O3(s)+ 6HF(l) 2BF3(g)+ 6H2O(l)
B2O3(s)+ HF(l) BF3(g)+ H2O(l)
A)B2O3(s)+ 6HF(l) 2BF3(g)+ 3H2O(l)
B)B2O3(s)+ H6F6(l) B2F6(g)+ H6O3(l)
C)B2O3(s)+ 2HF(l) 2BF3(g)+ H2O(l)
D)B2O3(s)+ 3HF(l) 2BF3(g)+ 3H2O(l)
E)B2O3(s)+ 6HF(l) 2BF3(g)+ 6H2O(l)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
32
Terephthalic acid,used in the production of polyester fibers and films,is composed of carbon,hydrogen,and oxygen.When 0.6943 g of terephthalic acid was subjected to combustion analysis it produced 1.471 g CO2 and 0.226 g H2O.If its molar mass is between 158 and 167 g/mol,what is its molecular formula?
A)C4H6O7
B)C6H8O5
C)C7H12O4
D)C4H3O2
E)C8H6O4
A)C4H6O7
B)C6H8O5
C)C7H12O4
D)C4H3O2
E)C8H6O4

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
33
Balance the following equation for the combustion of benzene:
C6H6(l)+ O2(g) H2O(g)+ CO2(g)
A)C6H6(l)+ 9O2(g) 3H2O(g)+ 6CO2(g)
B)C6H6(l)+ 9O2(g) 6H2O(g)+ 6CO2(g)
C)2C6H6(l)+ 15O2(g) 6H2O(g)+ 12CO2(g)
D)C6H6(l)+ 15O2(g) 3H2O(g)+ 6CO2(g)
E)2C6H6(l)+ 9O2(g) 6H2O(g)+ 12CO2(g)
C6H6(l)+ O2(g) H2O(g)+ CO2(g)
A)C6H6(l)+ 9O2(g) 3H2O(g)+ 6CO2(g)
B)C6H6(l)+ 9O2(g) 6H2O(g)+ 6CO2(g)
C)2C6H6(l)+ 15O2(g) 6H2O(g)+ 12CO2(g)
D)C6H6(l)+ 15O2(g) 3H2O(g)+ 6CO2(g)
E)2C6H6(l)+ 9O2(g) 6H2O(g)+ 12CO2(g)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
34
Balance the following equation:
C8H18O3(l)+ O2(g) H2O(g)+ CO2(g)
A)C8H18O3(l)+ 8O2(g) 9H2O(g)+ 8CO2(g)
B)C8H18O3(l)+ 11O2(g) 9H2O(g)+ 8CO2(g)
C)2C8H18O3(l)+ 22O2(g) 9H2O(g)+ 16CO2(g)
D)C8H18O3(l)+ 13O2(g) 18H2O(g)+ 8CO2(g)
E)2C8H18O3(l)+ 17O2(g) 18H2O(g)+ 16CO2(g)
C8H18O3(l)+ O2(g) H2O(g)+ CO2(g)
A)C8H18O3(l)+ 8O2(g) 9H2O(g)+ 8CO2(g)
B)C8H18O3(l)+ 11O2(g) 9H2O(g)+ 8CO2(g)
C)2C8H18O3(l)+ 22O2(g) 9H2O(g)+ 16CO2(g)
D)C8H18O3(l)+ 13O2(g) 18H2O(g)+ 8CO2(g)
E)2C8H18O3(l)+ 17O2(g) 18H2O(g)+ 16CO2(g)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
35
Balance the following equation:
Ca3(PO4)2(s)+ SiO2(s)+ C(s) CaSiO3(s)+ CO(g)+ P4(s)
A)Ca3(PO4)2(s)+ 3SiO2(s)+ 8C(s) 3CaSiO3(s)+ 8CO(g)+ P4(s)
B)Ca3(PO4)2(s)+ 3SiO2(s)+ 14C(s) 3CaSiO3(s)+ 14CO(g)+ P4(s)
C)Ca3(PO4)2(s)+ 3SiO2(s)+ 8C(s) 3CaSiO3(s)+ 8CO(g)+ 2P4(s)
D)2Ca3(PO4)2(s)+ 6SiO2(s)+ 10C(s) 6CaSiO3(s)+ 10CO(g)+ P4(s)
E)2Ca3(PO4)2(s)+ 6SiO2(s)+ 10C(s) 6CaSiO3(s)+ 10CO(g)+ 4P4(s)
Ca3(PO4)2(s)+ SiO2(s)+ C(s) CaSiO3(s)+ CO(g)+ P4(s)
A)Ca3(PO4)2(s)+ 3SiO2(s)+ 8C(s) 3CaSiO3(s)+ 8CO(g)+ P4(s)
B)Ca3(PO4)2(s)+ 3SiO2(s)+ 14C(s) 3CaSiO3(s)+ 14CO(g)+ P4(s)
C)Ca3(PO4)2(s)+ 3SiO2(s)+ 8C(s) 3CaSiO3(s)+ 8CO(g)+ 2P4(s)
D)2Ca3(PO4)2(s)+ 6SiO2(s)+ 10C(s) 6CaSiO3(s)+ 10CO(g)+ P4(s)
E)2Ca3(PO4)2(s)+ 6SiO2(s)+ 10C(s) 6CaSiO3(s)+ 10CO(g)+ 4P4(s)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
36
How many grams of sodium fluoride (used in water fluoridation and manufacture of insecticides)are needed to form 485 g of sulfur tetrafluoride?
3SCl2(l)+ 4NaF(s) SF4(g)+ S2Cl2(l)+ 4NaCl(s)
A)1940 g
B)1510 g
C)754 g
D)205 g
E)51.3 g
3SCl2(l)+ 4NaF(s) SF4(g)+ S2Cl2(l)+ 4NaCl(s)
A)1940 g
B)1510 g
C)754 g
D)205 g
E)51.3 g

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
37
Alkanes are compounds of carbon and hydrogen with the general formula CnH2n+2.An alkane component of gasoline has a molar mass of between 125 and 130 g/mol.What is the value of n for this alkane?
A)4
B)9
C)10
D)13
E)14
A)4
B)9
C)10
D)13
E)14

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
38
In the combustion analysis of 0.1127 g of glucose (C6H12O6),what mass,in grams,of CO2 would be produced?
A)0.0451 g
B)0.0825 g
C)0.1652 g
D)0.4132 g
E)1.466 g
A)0.0451 g
B)0.0825 g
C)0.1652 g
D)0.4132 g
E)1.466 g

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
39
Hydroxylamine nitrate contains 29.17 mass % N,4.20 mass % H,and 66.63 mass O.If its molar mass is between 94 and 98 g/mol,what is its molecular formula?
A)NH2O5
B)N2H4O4
C)N3H3O3
D)N4H8O2
E)N2H2O4
A)NH2O5
B)N2H4O4
C)N3H3O3
D)N4H8O2
E)N2H2O4

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
40
Gadolinium oxide,a colorless powder which absorbs carbon dioxide from the air,contains 86.76 mass % Gd.Determine its empirical formula.
A)Gd2O3
B)Gd3O2
C)Gd3O4
D)Gd4O3
E)GdO
A)Gd2O3
B)Gd3O2
C)Gd3O4
D)Gd4O3
E)GdO

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
41
What is the percent yield for the reaction
PCl3(g)+ Cl2(g) PCl5(g)
If 119.3 g of PCl5 (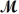
= 208.2 g/mol)are formed when 61.3 g of Cl2 (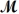
= 70.91 g/mol)react with excess PCl3?
A)195%
B)85.0%
C)66.3%
D)51.4%
E)43.7%
PCl3(g)+ Cl2(g) PCl5(g)
If 119.3 g of PCl5 (
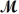
= 208.2 g/mol)are formed when 61.3 g of Cl2 (
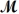
= 70.91 g/mol)react with excess PCl3?
A)195%
B)85.0%
C)66.3%
D)51.4%
E)43.7%

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
42
Aluminum metal reacts with chlorine gas to form solid aluminum trichloride,AlCl3.What mass of chlorine gas is needed to react completely with 163 g of aluminum?
A)214 g
B)245 g
C)321 g
D)489 g
E)643 g
A)214 g
B)245 g
C)321 g
D)489 g
E)643 g

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
43
Tetraphosphorus hexaoxide ( 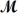
= 219.9 g/mol)is formed by the reaction of phosphorus with oxygen gas.
P4(s)+ 3O2(g) P4O6(s)
If a mixture of 75.3 g of phosphorus and 38.7 g of oxygen produce 43.3 g of P4O6,what is the percent yield for the reaction?
A)57.5%
B)48.8%
C)38.0%
D)32.4%
E)16.3%
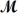
= 219.9 g/mol)is formed by the reaction of phosphorus with oxygen gas.
P4(s)+ 3O2(g) P4O6(s)
If a mixture of 75.3 g of phosphorus and 38.7 g of oxygen produce 43.3 g of P4O6,what is the percent yield for the reaction?
A)57.5%
B)48.8%
C)38.0%
D)32.4%
E)16.3%

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
44
Aluminum reacts with oxygen to produce aluminum oxide which can be used as an adsorbent,desiccant or catalyst for organic reactions.
4Al(s)+ 3O2(g) 2Al2O3(s)
A mixture of 82.49 g of aluminum (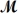
= 26.98 g/mol)and 117.65 g of oxygen (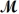
= 32.00 g/mol)is allowed to react.Identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete.
A)Oxygen is the limiting reactant;19.81 g of aluminum remain.
B)Oxygen is the limiting reactant;35.16 g of aluminum remain.
C)Aluminum is the limiting reactant;16.70 g of oxygen remain.
D)Aluminum is the limiting reactant;35.16 g of oxygen remain.
E)Aluminum is the limiting reactant;44.24 g of oxygen remain.
4Al(s)+ 3O2(g) 2Al2O3(s)
A mixture of 82.49 g of aluminum (
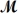
= 26.98 g/mol)and 117.65 g of oxygen (
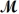
= 32.00 g/mol)is allowed to react.Identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete.
A)Oxygen is the limiting reactant;19.81 g of aluminum remain.
B)Oxygen is the limiting reactant;35.16 g of aluminum remain.
C)Aluminum is the limiting reactant;16.70 g of oxygen remain.
D)Aluminum is the limiting reactant;35.16 g of oxygen remain.
E)Aluminum is the limiting reactant;44.24 g of oxygen remain.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
45
Lead(II)sulfide was once used in glazing earthenware.It will also react with hydrogen peroxide to form lead(II)sulfate and water.How many grams of hydrogen peroxide are needed to react completely with 265 g of lead(II)sulfide?
A)151 g
B)123 g
C)50.3 g
D)37.7 g
E)9.41 g
A)151 g
B)123 g
C)50.3 g
D)37.7 g
E)9.41 g

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
46
Magnesium reacts with iron(III)chloride to form magnesium chloride (which can be used in fireproofing wood and in disinfectants)and iron.
3Mg(s)+ 2FeCl3(s) 3MgCl2(s)+ 2Fe(s)
A mixture of 41.0 g of magnesium (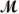
= 24.31 g/mol)and 175 g of iron(III)chloride (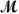
= 162.2 g/mol)is allowed to react.What mass of magnesium chloride = 95.21 g/mol)is formed?
A)68.5 g MgCl2
B)77.0 g MgCl2
C)71.4 g MgCl2
D)107 g MgCl2
E)154 g MgCl2
3Mg(s)+ 2FeCl3(s) 3MgCl2(s)+ 2Fe(s)
A mixture of 41.0 g of magnesium (
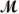
= 24.31 g/mol)and 175 g of iron(III)chloride (
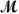
= 162.2 g/mol)is allowed to react.What mass of magnesium chloride = 95.21 g/mol)is formed?
A)68.5 g MgCl2
B)77.0 g MgCl2
C)71.4 g MgCl2
D)107 g MgCl2
E)154 g MgCl2

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
47
Methanol (CH4O)is converted to bromomethane (CH3Br)as follows:
CH4O + HBr CH3Br + H2O
If 12.23 g of bromomethane are produced when 5.00 g of methanol is reacted with excess HBr,what is the percentage yield?
A)40.9%
B)82.6%
C)100.%
D)121%
E)245%
CH4O + HBr CH3Br + H2O
If 12.23 g of bromomethane are produced when 5.00 g of methanol is reacted with excess HBr,what is the percentage yield?
A)40.9%
B)82.6%
C)100.%
D)121%
E)245%

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
48
Phosphine,an extremely poisonous and highly reactive gas,will react with oxygen to form tetraphosphorus decaoxide and water.
PH3(g)+ O2(g) P4O10(s)+ H2O(g)[unbalanced]
Calculate the mass of P4O10(s)formed when 225 g of PH3 reacts with excess oxygen.
A)1880 g
B)940.g
C)900.g
D)470 g
E)56.3 g
PH3(g)+ O2(g) P4O10(s)+ H2O(g)[unbalanced]
Calculate the mass of P4O10(s)formed when 225 g of PH3 reacts with excess oxygen.
A)1880 g
B)940.g
C)900.g
D)470 g
E)56.3 g

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
49
Potassium chlorate (used in fireworks,flares and safety matches)forms oxygen and potassium chloride when heated.
KClO3(s) KCl(s)+ O2(g)[unbalanced]
How many grams of oxygen are formed when 26.4 g of potassium chlorate is heated?
A)223 g
B)99.1 g
C)10.3 g
D)6.86 g
E)4.60 g
KClO3(s) KCl(s)+ O2(g)[unbalanced]
How many grams of oxygen are formed when 26.4 g of potassium chlorate is heated?
A)223 g
B)99.1 g
C)10.3 g
D)6.86 g
E)4.60 g

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
50
Sodium chlorate is used as an oxidizer in the manufacture of dyes,explosives and matches.Calculate the mass of solute needed to prepare 1.575 L of 0.00250 M NaClO3 ( 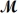
= 106.45 g/mol).
A)419 g
B)169 g
C)0.419 g
D)0.169 g
E)0.00394 g
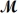
= 106.45 g/mol).
A)419 g
B)169 g
C)0.419 g
D)0.169 g
E)0.00394 g

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
51
A 0.150 M sodium chloride solution is referred to as a physiological saline solution because it has the same concentration of salts as normal human blood.Calculate the mass of solute needed to prepare 275.0 mL of a physiological saline solution.
A)41.3 g
B)31.9 g
C)16.1 g
D)8.77 g
E)2.41 g
A)41.3 g
B)31.9 g
C)16.1 g
D)8.77 g
E)2.41 g

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
52
Lithium hydroxide is used in alkaline batteries.Calculate the molarity of a solution prepared by dissolving 1.495 moles of LiOH in enough water to give a final volume of 750.mL.
A)1.99 M
B)1.50 M
C)1.12 M
D)0.502 M
E)0.00199 M
A)1.99 M
B)1.50 M
C)1.12 M
D)0.502 M
E)0.00199 M

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
53
How many grams of oxygen are needed to react completely with 200.0 g of ammonia,NH3?
4NH3(g)+ 5O2(g) 4NO(g)+ 6H2O(g)
A)469.7 g
B)300.6 g
C)250.0 g
D)3.406 g
E)2.180 g
4NH3(g)+ 5O2(g) 4NO(g)+ 6H2O(g)
A)469.7 g
B)300.6 g
C)250.0 g
D)3.406 g
E)2.180 g

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
54
Sodium hydroxide,also known as caustic soda,is used to neutralize acids and to treat cellulose in making of cellophane.Calculate the number of moles of solute in 1.875 L of 1.356 M NaOH solution.
A)2.543 mol
B)1.383 mol
C)0.7232 mol
D)0.3932 mol
E)0.001383 mol
A)2.543 mol
B)1.383 mol
C)0.7232 mol
D)0.3932 mol
E)0.001383 mol

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
55
Aluminum oxide (used as an adsorbent or a catalyst for organic reactions)forms when aluminum reacts with oxygen.
4Al(s)+ 3O2(g) 2Al2O3(s)
A mixture of 82.49 g of aluminum (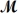
= 26.98 g/mol)and 117.65 g of oxygen (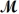
= 32.00 g/mol)is allowed to react.What mass of aluminum oxide (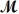
= 101.96 g/mol)can be formed?
A)155.8 g
B)200.2 g
C)249.9 g
D)311.7 g
E)374.9 g
4Al(s)+ 3O2(g) 2Al2O3(s)
A mixture of 82.49 g of aluminum (
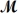
= 26.98 g/mol)and 117.65 g of oxygen (
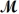
= 32.00 g/mol)is allowed to react.What mass of aluminum oxide (
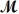
= 101.96 g/mol)can be formed?
A)155.8 g
B)200.2 g
C)249.9 g
D)311.7 g
E)374.9 g

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
56
Potassium chloride is used as a substitute for sodium chloride for individuals with high blood pressure.Identify the limiting reactant and determine the mass of the excess reactant remaining when 7.00 g of chlorine gas reacts with 5.00 g of potassium to form potassium chloride.
A)Potassium is the limiting reactant;2.47 g of chlorine remain.
B)Potassium is the limiting reactant;7.23 g of chlorine remain.
C)Chlorine is the limiting reactant;4.64 g of potassium remain.
D)Chlorine is the limiting reactant;2.70 g of potassium remain.
E)No limiting reagent: the reactants are present in the correct stoichiometric ratio.
A)Potassium is the limiting reactant;2.47 g of chlorine remain.
B)Potassium is the limiting reactant;7.23 g of chlorine remain.
C)Chlorine is the limiting reactant;4.64 g of potassium remain.
D)Chlorine is the limiting reactant;2.70 g of potassium remain.
E)No limiting reagent: the reactants are present in the correct stoichiometric ratio.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
57
Hydrochloric acid is widely used as a laboratory reagent,in refining ore for the production of tin and tantalum,and as a catalyst in organic reactions.Calculate the number of moles of HCl in 62.85 mL of 0.453 M hydrochloric acid.
A)28.5 mol
B)1.04 mol
C)0.139 mol
D)0.0285 mol
E)0.00721 mol
A)28.5 mol
B)1.04 mol
C)0.139 mol
D)0.0285 mol
E)0.00721 mol

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
58
When 2.61 g of solid Na2CO3 is dissolved in sufficient water to make 250 mL of solution,the concentration of Na2CO3 is:
A)0.0246 M
B)10.4 M
C)0.205 M
D)0.0985 M
E)0.141 M
A)0.0246 M
B)10.4 M
C)0.205 M
D)0.0985 M
E)0.141 M

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
59
Calculate the molarity of a 23.55-mL solution which contains 28.24 mg of sodium sulfate (used in dyeing and printing textiles, 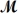
= 139.04 g/mol).
A)8.625 M
B)1.199 M
C)0.8339 M
D)0.2031 M
E)0.008625 M
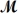
= 139.04 g/mol).
A)8.625 M
B)1.199 M
C)0.8339 M
D)0.2031 M
E)0.008625 M

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
60
Magnesium (used in the manufacture of light alloys)reacts with iron(III)chloride to form magnesium chloride and iron.
3Mg(s)+ 2FeCl3(s) 3MgCl2(s)+ 2Fe(s)
A mixture of 41.0 g of magnesium (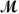
= 24.31 g/mol)and 175 g of iron(III)chloride (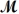
= 162.2 g/mol)is allowed to react.Identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete.
A)Limiting reactant is Mg;67 g of FeCl3 remain.
B)Limiting reactant is Mg;134 g of FeCl3 remain.
C)Limiting reactant is Mg;104 g of FeCl3 remain.
D)Limiting reactant is FeCl3;2 g of Mg remain.
E)Limiting reactant is FeCl3;87 g of Mg remain.
3Mg(s)+ 2FeCl3(s) 3MgCl2(s)+ 2Fe(s)
A mixture of 41.0 g of magnesium (
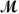
= 24.31 g/mol)and 175 g of iron(III)chloride (
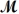
= 162.2 g/mol)is allowed to react.Identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete.
A)Limiting reactant is Mg;67 g of FeCl3 remain.
B)Limiting reactant is Mg;134 g of FeCl3 remain.
C)Limiting reactant is Mg;104 g of FeCl3 remain.
D)Limiting reactant is FeCl3;2 g of Mg remain.
E)Limiting reactant is FeCl3;87 g of Mg remain.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
61
Copper(II)sulfide,CuS,is used in the development of aniline black dye in textile printing.What is the maximum mass of CuS which can be formed when 38.0 mL of 0.500 M CuCl2 are mixed with 42.0 mL of 0.600 M (NH4)2S? Aqueous ammonium chloride is the other product.
A)2.41 g
B)1.82 g
C)1.21 g
D)0.909 g
E)0.044 g
A)2.41 g
B)1.82 g
C)1.21 g
D)0.909 g
E)0.044 g

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
62
Balance the following equation for the combustion of butane,a hydrocarbon used in gas lighters:
C4H10(g)+ O2(g) CO2(g)+ H2O(l)
C4H10(g)+ O2(g) CO2(g)+ H2O(l)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
63
Gaseous methanol (CH4O)reacts with oxygen gas to produce carbon dioxide gas and liquid water.Write a balanced equation for this process.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
64
Analysis of a white solid produced in a reaction between chlorine and phosphorus showed that it contained 77.44% chlorine and 22.56% phosphorus.What is its empirical formula?

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
65
For a sample consisting of 2.50 g of methane,CH4,calculate
a.the number of moles of methane present.
b.the total number of atoms present.
a.the number of moles of methane present.
b.the total number of atoms present.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
66
Ammonia,NH3 ,is produced industrially from nitrogen and hydrogen as follows:
N2(g)+ 3H2(g) 2NH3(g)
What mass,of which starting material,will remain when 30.0 g of N2 and 10.0 g of H2 react until the limiting reagent is completely consumed?
N2(g)+ 3H2(g) 2NH3(g)
What mass,of which starting material,will remain when 30.0 g of N2 and 10.0 g of H2 react until the limiting reagent is completely consumed?

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
67
Calcium chloride is used to melt ice and snow on roads and sidewalks and to remove water from organic liquids.Calculate the molarity of a solution prepared by diluting 165 mL of 0.688 M calcium chloride to 925.0 mL.
A)3.86 M
B)0.743 M
C)0.222 M
D)0.123 M
E)0.114 M
A)3.86 M
B)0.743 M
C)0.222 M
D)0.123 M
E)0.114 M

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
68
Consider the balanced equation:
Al2S3(s)+ 6H2O(l) 2Al(OH)3(s)+ 3H2S(g)
If 15.0g of aluminum sulfide and 10.0g of water are allowed to react as above,and assuming a complete reaction,
a.by calculation,find out which is the limiting reagent.
b.calculate the maximum mass of H2S which can be formed from these reagents.
c.calculate the mass of excess reagent remaining after the reaction is complete.
Al2S3(s)+ 6H2O(l) 2Al(OH)3(s)+ 3H2S(g)
If 15.0g of aluminum sulfide and 10.0g of water are allowed to react as above,and assuming a complete reaction,
a.by calculation,find out which is the limiting reagent.
b.calculate the maximum mass of H2S which can be formed from these reagents.
c.calculate the mass of excess reagent remaining after the reaction is complete.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
69
How many mL of concentrated nitric acid (HNO3,16.0 M)should be diluted with water in order to make 2.00 L of 2.00 M solution?
A)32.0 mL
B)62.5 mL
C)125 mL
D)250.mL
E)500.mL
A)32.0 mL
B)62.5 mL
C)125 mL
D)250.mL
E)500.mL

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
70
You are provided with a 250 mL volumetric flask,deionized water and solid NaOH.How much NaOH should be weighed out in order to make 250.mL of 0.100 M solution?

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
71
What will be the final volume of a solution prepared by diluting 25 mL of 8.25 M sodium hydroxide to a concentration of 2.40 M?
A)330 mL
B)210 mL
C)86 mL
D)60 mL
E)7.3 mL
A)330 mL
B)210 mL
C)86 mL
D)60 mL
E)7.3 mL

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
72
Propane,C3H8,is commonly provided as a bottled gas for use as a fuel.In 0.200 mol of propane,
A)what is the mass of propane?
B)7.21 g
C)1.20 1023 C3H8 molecules
D)9.64 1023 H atoms
A)what is the mass of propane?
B)7.21 g
C)1.20 1023 C3H8 molecules
D)9.64 1023 H atoms

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
73
How many milliliters of 1.58 M HCl are needed to react completely with 23.2 g of NaHCO3 ( 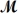
= 84.02 g/mol)?
HCl(aq)+ NaHCO3(s) NaCl(s)+ H2O(l)+ CO2(g)
A)638 mL
B)572 mL
C)536 mL
D)276 mL
E)175 mL
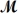
= 84.02 g/mol)?
HCl(aq)+ NaHCO3(s) NaCl(s)+ H2O(l)+ CO2(g)
A)638 mL
B)572 mL
C)536 mL
D)276 mL
E)175 mL

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
74
A compound consisting of C,H and O only,has a molar mass of 331.5 g/mol.Combustion of 0.1000 g of this compound caused a 0.2921 g increase in the mass of the CO2 absorber and a 0.0951 g increase in the mass of the H2O absorber.What is the empirical formula of the compound?

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
75
Consider the balanced equation for the combustion of propane,C3H8
C3H8(g)+ 5O2(g) 3CO2(g)+ 4H2O(l)
If propane reacts with oxygen as above,
a.what is the limiting reagent in a mixture containing 5.00 g of C3H8 and 10.0 g of O2?
b.what mass of CO2 is formed when 1.00 g of C3H8 reacts completely?
C3H8(g)+ 5O2(g) 3CO2(g)+ 4H2O(l)
If propane reacts with oxygen as above,
a.what is the limiting reagent in a mixture containing 5.00 g of C3H8 and 10.0 g of O2?
b.what mass of CO2 is formed when 1.00 g of C3H8 reacts completely?

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
76
Balance the following equation for partial oxidation of ammonia,an important reaction in the production of nitric acid:
NH3(g)+ O2(g) NO(g)+ H2O(l)
NH3(g)+ O2(g) NO(g)+ H2O(l)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
77
The insecticide DDT was formerly in widespread use,but now it is severely restricted owing to its adverse environmental effects.It is prepared as follows:

If 10.00 g of chloral were reacted with 10.00 g of chlorobenzene,
a.what is the maximum amount (mol)of DDT which could be formed?
b.what is the limiting reagent?
c.what is the % yield,if 12.15 g of DDT is produced?

If 10.00 g of chloral were reacted with 10.00 g of chlorobenzene,
a.what is the maximum amount (mol)of DDT which could be formed?
b.what is the limiting reagent?
c.what is the % yield,if 12.15 g of DDT is produced?

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
78
Balance the equation
B2O3(s)+ NaOH(aq) Na3BO3(aq)+ H2O(l)
B2O3(s)+ NaOH(aq) Na3BO3(aq)+ H2O(l)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
79
In 0.20 mole of phosphoric acid,H3PO4,
a.how many H atoms are there?
b.what is the total number of atoms?
c.how many moles of O atoms are there?
a.how many H atoms are there?
b.what is the total number of atoms?
c.how many moles of O atoms are there?

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
80
What volume,in L,of 10.0 M HCl is needed to make 2.00 L of 2.00 M HCl solution by dilution with water?
A)0.800 L
B)0.400 L
C)0.200 L
D)0.100 L
E)None of these choices is correct.
A)0.800 L
B)0.400 L
C)0.200 L
D)0.100 L
E)None of these choices is correct.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck


