Exam 3: Stoichiometry of Formulas and Equations
Exam 1: Keys to the Study of Chemistry66 Questions
Exam 2: The Components of Matter91 Questions
Exam 3: Stoichiometry of Formulas and Equations90 Questions
Exam 4: Three Major Classes of Chemical Reactions84 Questions
Exam 5: Gases and the Kinetic-Molecular Theory93 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change71 Questions
Exam 7: Quantum Theory and Atomic Structure72 Questions
Exam 8: Electron Configuration and Chemical Periodicity70 Questions
Exam 9: Models of Chemical Bonding60 Questions
Exam 10: The Shapes of Molecules94 Questions
Exam 11: Theories of Covalent Bonding49 Questions
Exam 12: Intermolecular Forces: Liquids,solids,and Phase Changes89 Questions
Exam 13: The Properties of Solutions73 Questions
Exam 14: The Main-Group Elements: Applying Principles of Bonding and Structure58 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon95 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions76 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions85 Questions
Exam 18: Acid-Base Equilibria90 Questions
Exam 19: Ionic Equilibria in Aqueous Systems96 Questions
Exam 20: Thermodynamics: Entropy, free Energy, and the Direction of Chemical Reactions84 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work97 Questions
Exam 22: The Transition Elements and Their Coordination Compounds72 Questions
Exam 23: Nuclear Reactions and Their Applications75 Questions
Select questions type
Balance the following equation:
Ca3(PO4)2(s)+ SiO2(s)+ C(s) CaSiO3(s)+ CO(g)+ P4(s)
Free
(Multiple Choice)
4.9/5  (30)
(30)
Correct Answer:
D
Balance the equation
B2O3(s)+ NaOH(aq) Na3BO3(aq)+ H2O(l)
Free
(Essay)
4.9/5  (37)
(37)
Correct Answer:
B2O3(s)+ 6NaOH(aq) 2Na3BO3(aq)+ 3H2O(l)
Potassium chloride is used as a substitute for sodium chloride for individuals with high blood pressure.Identify the limiting reactant and determine the mass of the excess reactant remaining when 7.00 g of chlorine gas reacts with 5.00 g of potassium to form potassium chloride.
Free
(Multiple Choice)
4.9/5  (34)
(34)
Correct Answer:
A
Aluminum metal dissolved in hydrochloric acid as follows:
2Al(s)+ 6HCl(aq) 2AlCl3(aq)+ 3H2(g)
a.What is the minimum volume of 6.0 M HCl(aq)needed to completely dissolve 3.20 g of aluminum in this reaction?
b.What mass of AlCl3 would be produced by complete reaction of 3.20 g of aluminum?
(Essay)
4.7/5  (44)
(44)
Sodium bromate is used in a mixture which dissolves gold from its ores.Calculate the mass in grams of 4.68 mol of sodium bromate.
(Multiple Choice)
4.9/5  (31)
(31)
Ammonia,NH3 ,is produced industrially from nitrogen and hydrogen as follows:
N2(g)+ 3H2(g) 2NH3(g)
What mass,of which starting material,will remain when 30.0 g of N2 and 10.0 g of H2 react until the limiting reagent is completely consumed?
(Short Answer)
4.9/5  (38)
(38)
A compound containing chromium and silicon contains 73.52 mass percent chromium.Determine its empirical formula.
(Multiple Choice)
4.8/5  (36)
(36)
Aluminum sulfate,Al2(SO4)3,is used in tanning leather,purifying water,and manufacture of antiperspirants.Calculate its molar mass.
(Multiple Choice)
4.8/5  (36)
(36)
Balance the following equation for the combustion of benzene:
C6H6(l)+ O2(g) H2O(g)+ CO2(g)
(Multiple Choice)
4.7/5  (37)
(37)
Copper(II)sulfide,CuS,is used in the development of aniline black dye in textile printing.What is the maximum mass of CuS which can be formed when 38.0 mL of 0.500 M CuCl2 are mixed with 42.0 mL of 0.600 M (NH4)2S? Aqueous ammonium chloride is the other product.
(Multiple Choice)
4.7/5  (44)
(44)
What is the mass in grams of 0.250 mol of the common antacid calcium carbonate?
(Multiple Choice)
4.9/5  (38)
(38)
A solution of methanol (CH4O)in water has a concentration of 0.200 M.What mass of methanol,in grams,is present in 0.150 liters of this solution?
(Short Answer)
4.9/5  (44)
(44)
Aluminum reacts with oxygen to produce aluminum oxide which can be used as an adsorbent,desiccant or catalyst for organic reactions.
4Al(s)+ 3O2(g) 2Al2O3(s)
A mixture of 82.49 g of aluminum ( 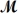 = 26.98 g/mol)and 117.65 g of oxygen (
= 26.98 g/mol)and 117.65 g of oxygen ( 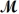 = 32.00 g/mol)is allowed to react.Identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete.
= 32.00 g/mol)is allowed to react.Identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete.
(Multiple Choice)
4.9/5  (40)
(40)
A 0.150 M sodium chloride solution is referred to as a physiological saline solution because it has the same concentration of salts as normal human blood.Calculate the mass of solute needed to prepare 275.0 mL of a physiological saline solution.
(Multiple Choice)
4.9/5  (34)
(34)
Sodium chlorate is used as an oxidizer in the manufacture of dyes,explosives and matches.Calculate the mass of solute needed to prepare 1.575 L of 0.00250 M NaClO3 ( 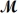 = 106.45 g/mol).
= 106.45 g/mol).
(Multiple Choice)
4.9/5  (38)
(38)
Hydrochloric acid is widely used as a laboratory reagent,in refining ore for the production of tin and tantalum,and as a catalyst in organic reactions.Calculate the number of moles of HCl in 62.85 mL of 0.453 M hydrochloric acid.
(Multiple Choice)
4.8/5  (38)
(38)
Calculate the mass in grams of 8.35 1022 molecules of CBr4.
(Multiple Choice)
4.8/5  (38)
(38)
How many grams of sodium fluoride (used in water fluoridation and manufacture of insecticides)are needed to form 485 g of sulfur tetrafluoride?
3SCl2(l)+ 4NaF(s) SF4(g)+ S2Cl2(l)+ 4NaCl(s)
(Multiple Choice)
4.9/5  (34)
(34)
Ammonia,an important source of fixed nitrogen that can be metabolized by plants,is produced using the Haber process in which nitrogen and hydrogen combine.
N2(g)+ 3H2(g) 2NH3(g)
How many grams of nitrogen are needed to produce 325 grams of ammonia?
(Multiple Choice)
5.0/5  (43)
(43)
Showing 1 - 20 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)