Deck 7: Quantum Theory and Atomic Structure
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/72
Play
Full screen (f)
Deck 7: Quantum Theory and Atomic Structure
1
Who proposed a model that successfully explained the photoelectric effect?
A)Planck
B)Einstein
C)Compton
D)Rydberg
E)Bohr
A)Planck
B)Einstein
C)Compton
D)Rydberg
E)Bohr
Einstein
2
The AM station KBOR plays your favorite music from the 20s and 30s at 1290 kHz.Find the wavelength of these waves.
A)4.30 10-2 m
B)0.144 m
C)6.94 m
D)232 m
E)> 103 m
A)4.30 10-2 m
B)0.144 m
C)6.94 m
D)232 m
E)> 103 m
232 m
3
Who developed an empirical equation from which the wavelengths of lines in the spectrum of hydrogen atoms can be calculated?
A)Planck
B)de Broglie
C)Bohr
D)Rutherford
E)Rydberg
A)Planck
B)de Broglie
C)Bohr
D)Rutherford
E)Rydberg
Rydberg
4
Select the arrangement of electromagnetic radiation which starts with the lowest wavelength and increases to greatest wavelength.
A)radio,infrared,ultraviolet,gamma rays
B)radio,ultraviolet,infrared,gamma rays
C)gamma rays,radio,ultraviolet,infrared
D)gamma rays,infrared,radio,ultraviolet
E)gamma rays,ultraviolet,infrared,radio
A)radio,infrared,ultraviolet,gamma rays
B)radio,ultraviolet,infrared,gamma rays
C)gamma rays,radio,ultraviolet,infrared
D)gamma rays,infrared,radio,ultraviolet
E)gamma rays,ultraviolet,infrared,radio

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
5
Which word best describes the phenomenon which gives rise to a rainbow?
A)reflection
B)dispersion
C)diffraction
D)interference
E)deflection
A)reflection
B)dispersion
C)diffraction
D)interference
E)deflection

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
6
The FM station KDUL broadcasts music at 99.1 MHz.Find the wavelength of these waves.
A)1.88 10-2 m
B)0.330 m
C)3.03 m
D)5.33 102 m
E)> 103 m
A)1.88 10-2 m
B)0.330 m
C)3.03 m
D)5.33 102 m
E)> 103 m

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
7
Who was the first scientist to propose that an object could emit only certain amounts of energy?
A)Planck
B)Einstein
C)Bohr
D)Rydberg
E)de Broglie
A)Planck
B)Einstein
C)Bohr
D)Rydberg
E)de Broglie

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
8
Interference of light waves
A)separates light into its component colors.
B)creates a pattern of light and dark regions.
C)focuses a broad beam of light into a point.
D)bends light as it passes the edge of an object.
E)creates a laser beam.
A)separates light into its component colors.
B)creates a pattern of light and dark regions.
C)focuses a broad beam of light into a point.
D)bends light as it passes the edge of an object.
E)creates a laser beam.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
9
A radio wave has a frequency of 8.6 108 Hz.What is the energy of one photon of this radiation?
A)7.7 10-43 J
B)2.3 10-34 J
C)5.7 10-25 J
D)1.7 10-16 J
E)> 10-15 J
A)7.7 10-43 J
B)2.3 10-34 J
C)5.7 10-25 J
D)1.7 10-16 J
E)> 10-15 J

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
10
Who proposed the principle which states that one cannot simultaneously know the exact position and velocity of a particle?
A)Einstein
B)Planck
C)Heisenberg
D)Compton
E)de Broglie
A)Einstein
B)Planck
C)Heisenberg
D)Compton
E)de Broglie

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
11
An infrared wave has a wavelength of 6.5 10-4 cm.What is this distance in angstroms,Å?
A)6.5 10-4 Å
B)2.2 10-4 Å
C)4.6 103 Å
D)6.5 104 Å
E)6.5 106 Å
A)6.5 10-4 Å
B)2.2 10-4 Å
C)4.6 103 Å
D)6.5 104 Å
E)6.5 106 Å

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
12
Contact lenses can focus light due to the ___________ of the waves.
A)diffraction
B)reflection
C)refraction
D)dispersion
E)interference
A)diffraction
B)reflection
C)refraction
D)dispersion
E)interference

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
13
Who was the first scientist to propose that the atom had a dense nucleus which occupied only a small fraction of the volume of the atom?
A)Planck
B)Bohr
C)Rydberg
D)Rutherford
E)Thomson
A)Planck
B)Bohr
C)Rydberg
D)Rutherford
E)Thomson

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
14
Which scientist demonstrated that photons transferred momentum during collisions with matter?
A)Bohr
B)de Broglie
C)Planck
D)Compton
E)Billiard
A)Bohr
B)de Broglie
C)Planck
D)Compton
E)Billiard

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
15
Select the arrangement of electromagnetic radiation which starts with the lowest energy and increases to greatest energy.
A)radio,infrared,ultraviolet,gamma rays
B)radio,ultraviolet,infrared,gamma rays
C)gamma rays,infrared,radio,ultraviolet
D)gamma rays,ultraviolet,infrared,radio
E)infrared,ultraviolet,radio,gamma rays
A)radio,infrared,ultraviolet,gamma rays
B)radio,ultraviolet,infrared,gamma rays
C)gamma rays,infrared,radio,ultraviolet
D)gamma rays,ultraviolet,infrared,radio
E)infrared,ultraviolet,radio,gamma rays

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
16
Select the arrangement of electromagnetic radiation which starts with the lowest energy and increases to greatest energy.
A)radio,visible,infrared,ultraviolet
B)infrared,visible,ultraviolet,microwave
C)visible,ultraviolet,infrared,gamma rays
D)X-radiation,visible,infrared,microwave
E)microwave,infrared,visible,ultraviolet
A)radio,visible,infrared,ultraviolet
B)infrared,visible,ultraviolet,microwave
C)visible,ultraviolet,infrared,gamma rays
D)X-radiation,visible,infrared,microwave
E)microwave,infrared,visible,ultraviolet

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
17
The interference pattern seen when light passes through narrow,closely spaced slits,is due to
A)diffraction.
B)reflection.
C)refraction.
D)dispersion.
E)deflection.
A)diffraction.
B)reflection.
C)refraction.
D)dispersion.
E)deflection.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
18
Which scientist first proposed that particles of matter could have wave properties?
A)Einstein
B)Planck
C)de Broglie
D)Compton
E)Heisenberg
A)Einstein
B)Planck
C)de Broglie
D)Compton
E)Heisenberg

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
19
Electromagnetic radiation of 500 nm wavelength lies in the ________ region of the spectrum.
A)infrared
B)visible
C)ultraviolet
D)X-ray
E)( -ray)
A)infrared
B)visible
C)ultraviolet
D)X-ray
E)( -ray)

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
20
Which scientist first proposed that the electron in the hydrogen atom can have only certain energies?
A)Planck
B)Einstein
C)Bohr
D)Rydberg
E)Heisenberg
A)Planck
B)Einstein
C)Bohr
D)Rydberg
E)Heisenberg

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
21
An electron in the n = 6 level emits a photon with a wavelength of 410.2 nm.To what energy level does the electron move?
A)n = 1
B)n = 2
C)n = 3
D)n = 4
E)n = 5
A)n = 1
B)n = 2
C)n = 3
D)n = 4
E)n = 5

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
22
According to the Rydberg equation,the longest wavelength (in nm)in the series of H-atom lines with
N1 = 3 is
A)1875 nm
B)1458 nm
C)820 nm
D)656 nm
E)365 nm
N1 = 3 is
A)1875 nm
B)1458 nm
C)820 nm
D)656 nm
E)365 nm

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
23
A sprinter must average 24.0 mi/h to win a 100-m dash in 9.30 s.What is his wavelength at this speed if his mass is 84.5 kg?
A)7.29 10-37 m
B)3.26 10-37 m
C)5.08 10-30 m
D)1.34 10-30 m
E)None of these choices is correct.
A)7.29 10-37 m
B)3.26 10-37 m
C)5.08 10-30 m
D)1.34 10-30 m
E)None of these choices is correct.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
24
Green light has a wavelength of 5200 Å.Calculate the energy of one photon of green light.
A)3.4 10-40 J
B)3.4 10-30 J
C)3.8 10-29 J
D)3.4 10-27 J
E)3.8 10-19 J
A)3.4 10-40 J
B)3.4 10-30 J
C)3.8 10-29 J
D)3.4 10-27 J
E)3.8 10-19 J

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
25
Consider the following adjectives used to describe types of spectrum:
Continuous line atomic emission absorption
How many of them are appropriate to describe the spectrum of radiation given off by a black body?
A)none
B)one
C)two
D)three
E)four
Continuous line atomic emission absorption
How many of them are appropriate to describe the spectrum of radiation given off by a black body?
A)none
B)one
C)two
D)three
E)four

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
26
The Bohr theory of the hydrogen atom predicts the energy difference (in J)between the n = 3 and the n = 5 state to be
A)8.72 10-20 J
B)1.36 10-19 J
C)2.42 10-19 J
D)1.55 10-19 J
E)1.09 10-18 J
A)8.72 10-20 J
B)1.36 10-19 J
C)2.42 10-19 J
D)1.55 10-19 J
E)1.09 10-18 J

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
27
Infrared radiation from the sun has a wavelength of 6200 nm.Calculate the energy of one photon of that radiation.
A)4.l 10-39 J
B)4.l 10-30 J
C)3.2 10-29 J
D)3.2 10-26 J
E)between 10-20 and 10-19 J
A)4.l 10-39 J
B)4.l 10-30 J
C)3.2 10-29 J
D)3.2 10-26 J
E)between 10-20 and 10-19 J

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
28
The de Broglie equation predicts that the wavelength (in m)of a proton moving at 1000.m/s is
A)3.96 10-10 m
B)3.96 10-7 m
C)2.52 106 m
D)2.52 109 m
E)> 1010 m
A)3.96 10-10 m
B)3.96 10-7 m
C)2.52 106 m
D)2.52 109 m
E)> 1010 m

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
29
According to the Rydberg equation,the line with the shortest wavelength in the emission spectrum of atomic hydrogen is predicted to lie at a wavelength (in nm)of
A)91.2 nm
B)1.10 10-2 nm
C)1.10 102 nm
D)1.10 1016 nm
E)None of these choices is correct.
A)91.2 nm
B)1.10 10-2 nm
C)1.10 102 nm
D)1.10 1016 nm
E)None of these choices is correct.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
30
For potassium metal,the work function (the minimum energy needed to eject an electron from the metal surface)is 3.68 10-19 J.Which is the longest wavelength of the following which could excite photoelectrons?
A)550.nm
B)500.nm
C)450.nm
D)400.nm
E)350.nm
A)550.nm
B)500.nm
C)450.nm
D)400.nm
E)350.nm

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
31
According to the Bohr theory of the hydrogen atom,the minimum energy (in J)needed to ionize a hydrogen atom from the n = 2 state is
A)2.18 10-18 J
B)1.64 10-18 J
C)5.45 10-19 J
D)3.03 10-19 J
E)None of these choices is correct.
A)2.18 10-18 J
B)1.64 10-18 J
C)5.45 10-19 J
D)3.03 10-19 J
E)None of these choices is correct.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
32
A photon has an energy of 5.53 10-17 J.What is its frequency in s-1?
A)3.66 10-50 s-1
B)1.20 10-17 s-1
C)3.59 10-9 s-1
D)2.78 108 s-1
E)8.35 1016 s-1
A)3.66 10-50 s-1
B)1.20 10-17 s-1
C)3.59 10-9 s-1
D)2.78 108 s-1
E)8.35 1016 s-1

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
33
The size of an atomic orbital is associated with
A)the principal quantum number (n).
B)the angular momentum quantum number (l).
C)the magnetic quantum number (ml).
D)the spin quantum number (ms).
E)the angular momentum and magnetic quantum numbers,together.
A)the principal quantum number (n).
B)the angular momentum quantum number (l).
C)the magnetic quantum number (ml).
D)the spin quantum number (ms).
E)the angular momentum and magnetic quantum numbers,together.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
34
The orientation in space of an atomic orbital is associated with
A)the principal quantum number (n).
B)the angular momentum quantum number (l).
C)the magnetic quantum number (ml).
D)the spin quantum number (ms).
E)None of these choices is correct.
A)the principal quantum number (n).
B)the angular momentum quantum number (l).
C)the magnetic quantum number (ml).
D)the spin quantum number (ms).
E)None of these choices is correct.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
35
Consider the following adjectives used to describe types of spectrum:
Continuous line atomic emission absorption
How many of them are appropriate to describe the spectrum of radiation absorbed by a sample of mercury vapor?
A)one
B)two
C)three
D)four
E)five
Continuous line atomic emission absorption
How many of them are appropriate to describe the spectrum of radiation absorbed by a sample of mercury vapor?
A)one
B)two
C)three
D)four
E)five

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
36
Platinum,which is widely used as a catalyst,has a work function (the minimum energy needed to eject an electron from the metal surface)of 9.05 10-19 J.What is the longest wavelength of light which will cause electrons to be emitted?
A)2.196 10-7 m
B)4.553 10-6 m
C)5.654 102 m
D)1.370 1015 m
E)> 106 m
A)2.196 10-7 m
B)4.553 10-6 m
C)5.654 102 m
D)1.370 1015 m
E)> 106 m

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
37
Use the Rydberg equation to calculate the frequency of a photon absorbed when the hydrogen atom undergoes a transition from n1 = 2 to n2 = 4.(R = 1.096776 107 m-1)
A)2.056 106 s-1
B)2.742 106 s-1
C)6.165 1014 s-1
D)8.226 1014 s-1
E)> 1015 s-1
A)2.056 106 s-1
B)2.742 106 s-1
C)6.165 1014 s-1
D)8.226 1014 s-1
E)> 1015 s-1

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
38
Line spectra from all regions of the electromagnetic spectrum,including the Paschen series of infrared lines for hydrogen,are used by astronomers to identify elements present in the atmospheres of stars.Calculate the wavelength of the photon emitted when the hydrogen atom undergoes a transition from n = 5 to n = 3.(R = 1.096776 107 m-1)
A)205.1 nm
B)384.6 nm
C)683.8 nm
D)1282 nm
E)> 1500 nm
A)205.1 nm
B)384.6 nm
C)683.8 nm
D)1282 nm
E)> 1500 nm

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
39
The shape of an atomic orbital is associated with
A)the principal quantum number (n).
B)the angular momentum quantum number (l).
C)the magnetic quantum number (ml).
D)the spin quantum number (ms).
E)the magnetic and spin quantum numbers,together.
A)the principal quantum number (n).
B)the angular momentum quantum number (l).
C)the magnetic quantum number (ml).
D)the spin quantum number (ms).
E)the magnetic and spin quantum numbers,together.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
40
If the energy of a photon is 1.32 10-18 J,what is its wavelength in nm?
A)1.50 10-7 nm
B)150.nm
C)1.99 1015 nm
D)1.99 1024 nm
E)None of these choices is correct.
A)1.50 10-7 nm
B)150.nm
C)1.99 1015 nm
D)1.99 1024 nm
E)None of these choices is correct.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
41
In the quantum mechanical treatment of the hydrogen atom,which one of the following combinations of quantum numbers is not allowed? 
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
42
Atomic orbitals developed using quantum mechanics
A)describe regions of space in which one is most likely to find an electron.
B)describe exact paths for electron motion.
C)give a description of the atomic structure which is essentially the same as the Bohr model.
D)allow scientists to calculate an exact volume for the hydrogen atom.
E)are in conflict with the Heisenberg Uncertainty Principle.
A)describe regions of space in which one is most likely to find an electron.
B)describe exact paths for electron motion.
C)give a description of the atomic structure which is essentially the same as the Bohr model.
D)allow scientists to calculate an exact volume for the hydrogen atom.
E)are in conflict with the Heisenberg Uncertainty Principle.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
43
Use the Rydberg equation to calculate the wavelength,in nm,of the least energetic (longest wavelength)line in the visible series (n1 = 2)of the spectrum of atomic hydrogen.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
44
Use the Bohr equation to calculate the energy of
a.the largest energy absorption or emission process involving the n = 2 state of the hydrogen atom.
b.the smallest energy absorption or emission process involving the n = 2 state of the hydrogen atom.
a.the largest energy absorption or emission process involving the n = 2 state of the hydrogen atom.
b.the smallest energy absorption or emission process involving the n = 2 state of the hydrogen atom.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
45
a.Use the Bohr equation to calculate the energy needed to ionize a hydrogen atom from its ground state.
b.What is the minimum wavelength of a photon needed for it to have the energy needed in (a)?
b.What is the minimum wavelength of a photon needed for it to have the energy needed in (a)?

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
46
What are the possible values for the following quantum numbers in an atom?
a.n
b.l
c.ml
a.n
b.l
c.ml

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
47
Which one of the following sets of quantum numbers can correctly represent a 3p orbital? 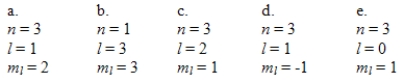
A)a
B)b
C)c
D)d
E)e
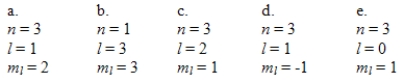
A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
48
a.Calculate the wavelength in nm of a photon whose energy is 6.00 10-19 J.
b.Would the photon in (a)have enough energy to ionize a hydrogen atom in its ground state (i.e. ,to separate the proton and electron completely)? Use the Bohr equation to explain your answer.
b.Would the photon in (a)have enough energy to ionize a hydrogen atom in its ground state (i.e. ,to separate the proton and electron completely)? Use the Bohr equation to explain your answer.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
49
What is the speed of an electron in m/s if its wavelength is 0.155 nm?

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
50
a.What is the frequency of microwave radiation which has a wavelength of 10.7 cm?
b.What is the energy of one photon of this radiation?
b.What is the energy of one photon of this radiation?

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
51
For the following equations,
a.name the scientist to whom the equation is attributed.
b.in not more than three lines,explain clearly what the equation means or represents.
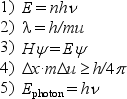
a.name the scientist to whom the equation is attributed.
b.in not more than three lines,explain clearly what the equation means or represents.
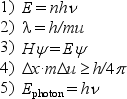

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following is a correct set of quantum numbers for an electron in a 3d orbital?
A)n = 3,l = 0,ml = -1
B)n = 3,l = 1,ml = +3
C)n = 3,l = 2,ml = 3
D)n = 3,l = 3,ml = +2
E)n = 3,l = 2,ml = -2
A)n = 3,l = 0,ml = -1
B)n = 3,l = 1,ml = +3
C)n = 3,l = 2,ml = 3
D)n = 3,l = 3,ml = +2
E)n = 3,l = 2,ml = -2

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
53
The following combinations of quantum numbers are not allowed.Correct each set by changing only one quantum number,and write in an appropriate corrected value.
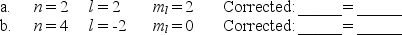
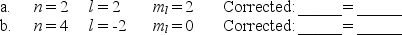

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
54
The energy of an electron in the hydrogen atom is determined by
A)the principal quantum number (n)only.
B)the angular momentum quantum number (l )only.
C)the principal and angular momentum quantum numbers (n & l ).
D)the principal and magnetic quantum numbers (n & ml).
E)the principal,angular momentum and magnetic quantum numbers.
A)the principal quantum number (n)only.
B)the angular momentum quantum number (l )only.
C)the principal and angular momentum quantum numbers (n & l ).
D)the principal and magnetic quantum numbers (n & ml).
E)the principal,angular momentum and magnetic quantum numbers.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
55
In not more than three lines for each answer,briefly outline one important scientific contribution of each of the following.
a.Planck
b.de Broglie
c.Heisenberg
a.Planck
b.de Broglie
c.Heisenberg

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
56
a.Use Bohr's equation to calculate how much energy (in J)is needed to promote an electron from the H-atom ground state to the n = 4 level.
b.If a photon provides the energy in (a),what is its wavelength in nm?
b.If a photon provides the energy in (a),what is its wavelength in nm?

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following is a correct set of quantum numbers for an electron in a 5f orbital?
A)n = 5,l = 3,ml = +1
B)n = 5,l = 2,ml = +3
C)n = 4,l = 3,ml = 0
D)n = 4,l = 2,ml = +1
E)n = 5,l = 4,ml = 3
A)n = 5,l = 3,ml = +1
B)n = 5,l = 2,ml = +3
C)n = 4,l = 3,ml = 0
D)n = 4,l = 2,ml = +1
E)n = 5,l = 4,ml = 3

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
58
a.Calculate the momentum of a photon of green light,wavelength 515 nm.
b.If this photon is traveling in a vacuum,what is its "mass"?
b.If this photon is traveling in a vacuum,what is its "mass"?

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
59
In the quantum mechanical treatment of the hydrogen atom,the functions and 2 both feature prominently.Briefly explain (in principle)how they are obtained and what,if anything,their physical meanings are.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
60
Explain the context and meanings of the terms "orbit" and "orbital",making a clear distinction between them.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
61
The Rydberg equation is an example of an empirical equation.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
62
For the following orbitals,state the values of n,l and ml which apply,and draw a sketch showing the shape and orientation of the orbital.
a.3s
b.2px
a.3s
b.2px

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
63
The Rydberg equation,giving the wavelengths of lines in the spectrum of the hydrogen atom,was obtained by assuming that energy is quantized.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
64
The energy of a photon is directly proportional to the wavelength of the radiation.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
65
In the quantum mechanical treatment of the hydrogen atom,the energy depends on the principal quantum number n but not on the values of l or ml.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
66
In the quantum mechanical treatment of the hydrogen atom,the probability of finding an electron at any point is proportional to the wave function .

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
67
Other factors being constant,a heavy object will have a longer de Broglie wavelength than a light object.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
68
In the Rydberg equation,for a fixed value of n1,the longest wavelength line has n2 =

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
69
Continuous spectra are characteristic of molecules in the gas phase.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
70
In the Bohr model of the hydrogen atom,the electron moves in a circular path which Bohr referred to as an orbital.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
71
Line spectra are characteristic of atoms in the gas phase.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
72
Continuous spectra are characteristic of heated solids.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck


