Exam 7: Quantum Theory and Atomic Structure
Exam 1: Keys to the Study of Chemistry66 Questions
Exam 2: The Components of Matter91 Questions
Exam 3: Stoichiometry of Formulas and Equations90 Questions
Exam 4: Three Major Classes of Chemical Reactions84 Questions
Exam 5: Gases and the Kinetic-Molecular Theory93 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change71 Questions
Exam 7: Quantum Theory and Atomic Structure72 Questions
Exam 8: Electron Configuration and Chemical Periodicity70 Questions
Exam 9: Models of Chemical Bonding60 Questions
Exam 10: The Shapes of Molecules94 Questions
Exam 11: Theories of Covalent Bonding49 Questions
Exam 12: Intermolecular Forces: Liquids,solids,and Phase Changes89 Questions
Exam 13: The Properties of Solutions73 Questions
Exam 14: The Main-Group Elements: Applying Principles of Bonding and Structure58 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon95 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions76 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions85 Questions
Exam 18: Acid-Base Equilibria90 Questions
Exam 19: Ionic Equilibria in Aqueous Systems96 Questions
Exam 20: Thermodynamics: Entropy, free Energy, and the Direction of Chemical Reactions84 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work97 Questions
Exam 22: The Transition Elements and Their Coordination Compounds72 Questions
Exam 23: Nuclear Reactions and Their Applications75 Questions
Select questions type
The Rydberg equation is an example of an empirical equation.
Free
(True/False)
4.7/5  (38)
(38)
Correct Answer:
True
Which word best describes the phenomenon which gives rise to a rainbow?
Free
(Multiple Choice)
4.9/5  (35)
(35)
Correct Answer:
B
Green light has a wavelength of 5200 Å.Calculate the energy of one photon of green light.
Free
(Multiple Choice)
4.9/5  (32)
(32)
Correct Answer:
E
Continuous spectra are characteristic of molecules in the gas phase.
(True/False)
4.9/5  (43)
(43)
Select the arrangement of electromagnetic radiation which starts with the lowest energy and increases to greatest energy.
(Multiple Choice)
4.9/5  (39)
(39)
A sprinter must average 24.0 mi/h to win a 100-m dash in 9.30 s.What is his wavelength at this speed if his mass is 84.5 kg?
(Multiple Choice)
4.9/5  (27)
(27)
Who proposed a model that successfully explained the photoelectric effect?
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following is a correct set of quantum numbers for an electron in a 3d orbital?
(Multiple Choice)
4.9/5  (37)
(37)
For potassium metal,the work function (the minimum energy needed to eject an electron from the metal surface)is 3.68 10-19 J.Which is the longest wavelength of the following which could excite photoelectrons?
(Multiple Choice)
4.8/5  (37)
(37)
In the Rydberg equation,for a fixed value of n1,the longest wavelength line has n2 =
(True/False)
4.8/5  (40)
(40)
The orientation in space of an atomic orbital is associated with
(Multiple Choice)
4.9/5  (31)
(31)
Who proposed the principle which states that one cannot simultaneously know the exact position and velocity of a particle?
(Multiple Choice)
4.7/5  (32)
(32)
The Bohr theory of the hydrogen atom predicts the energy difference (in J)between the n = 3 and the n = 5 state to be
(Multiple Choice)
4.7/5  (37)
(37)
Which one of the following sets of quantum numbers can correctly represent a 3p orbital? 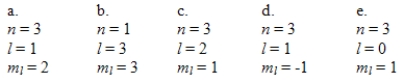
(Multiple Choice)
4.7/5  (32)
(32)
a.Calculate the momentum of a photon of green light,wavelength 515 nm.
b.If this photon is traveling in a vacuum,what is its "mass"?
(Essay)
5.0/5  (40)
(40)
In the quantum mechanical treatment of the hydrogen atom,which one of the following combinations of quantum numbers is not allowed? 
(Multiple Choice)
4.8/5  (31)
(31)
In the quantum mechanical treatment of the hydrogen atom,the functions and 2 both feature prominently.Briefly explain (in principle)how they are obtained and what,if anything,their physical meanings are.
(Essay)
4.9/5  (37)
(37)
In not more than three lines for each answer,briefly outline one important scientific contribution of each of the following.
a.Planck
b.de Broglie
c.Heisenberg
(Essay)
4.9/5  (35)
(35)
For the following equations,
a.name the scientist to whom the equation is attributed.
b.in not more than three lines,explain clearly what the equation means or represents.
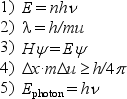
(Essay)
4.8/5  (38)
(38)
Showing 1 - 20 of 72
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)