Deck 3: Atoms and the Periodic Table
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/112
Play
Full screen (f)
Deck 3: Atoms and the Periodic Table
1
Which color of light carries the most energy per photon?
A) blue
B) green
C) orange
D) red
A) blue
B) green
C) orange
D) red
blue
2
In the atom represented by the symbol 23Na, there are
A) 11 protons, 11 electrons, 23 neutrons.
B) 12 protons, 12 electrons, 23 neutrons.
C) 11 protons, 11 electrons, 12 neutrons.
D) 12 protons, 12 electrons, 34 neutrons.
A) 11 protons, 11 electrons, 23 neutrons.
B) 12 protons, 12 electrons, 23 neutrons.
C) 11 protons, 11 electrons, 12 neutrons.
D) 12 protons, 12 electrons, 34 neutrons.
11 protons, 11 electrons, 12 neutrons.
3
Which combination of individual and contribution is not correct?
A) Antoine Lavoisier - clarified confusion over cause of burning
B) John Dalton - proposed atomic theory
C) Marie Curie - discovered radium and polonium
D) Robert Millikan - discovered the neutron
A) Antoine Lavoisier - clarified confusion over cause of burning
B) John Dalton - proposed atomic theory
C) Marie Curie - discovered radium and polonium
D) Robert Millikan - discovered the neutron
Robert Millikan - discovered the neutron
4
Who was the first person to propose a consistent modern atomic theory?
A) Democritus
B) Lavoisier
C) Proust
D) Dalton
A) Democritus
B) Lavoisier
C) Proust
D) Dalton

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following has the longest wavelength?
A) radio waves
B) X-rays
C) visible light
D) ultraviolet
A) radio waves
B) X-rays
C) visible light
D) ultraviolet

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
6
What is the main color of visible light emitted by neon?
A) yellow
B) orange-red
C) blue
D) green
A) yellow
B) orange-red
C) blue
D) green

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
7
What element is used to define the atomic mass unit scale?
A) hydrogen
B) oxygen
C) neon
D) carbon
A) hydrogen
B) oxygen
C) neon
D) carbon

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
8
What is the symbol for a neutral atom with 7 protons, 7 electrons, and 8 neutrons?
A) Si
B) P
C) Ne
D) N
A) Si
B) P
C) Ne
D) N

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
9
The term atom was first used by
A) Dalton.
B) Rutherford.
C) Democritus.
D) Lavoisier.
A) Dalton.
B) Rutherford.
C) Democritus.
D) Lavoisier.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
10
The third energy level can hold a maximum of how many electrons?
A) 0
B) 3
C) 9
D) 18
A) 0
B) 3
C) 9
D) 18

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
11
Which statement is true about the speed of photons and light waves?
A) Red light is slower than blue light.
B) Each color travels at a different speed.
C) All travel at the same speed.
D) The longer the wavelength is the lower the speed.
A) Red light is slower than blue light.
B) Each color travels at a different speed.
C) All travel at the same speed.
D) The longer the wavelength is the lower the speed.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
12
Which radiation type has no detectable charge?
A) alpha
B) beta
C) gamma
D) both alpha and beta
A) alpha
B) beta
C) gamma
D) both alpha and beta

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
13
Rutherford's foil experiment provided evidence for what atomic feature?
A) nucleus
B) electron
C) proton
D) neutron
A) nucleus
B) electron
C) proton
D) neutron

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is not included with a periodic table entry?
A) atomic number
B) mass number
C) atomic weight
D) symbol
A) atomic number
B) mass number
C) atomic weight
D) symbol

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
15
What is the number of valence electrons in phosphorus (P) with an electron configuration of 2-8-5?
A) 13
B) 8
C) 5
D) 15
A) 13
B) 8
C) 5
D) 15

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
16
Which electron configuration is inconsistent with the Bohr model?
A) 2-8-1
B) 2-6
C) 2-8-9
D) 2-8-8
A) 2-8-1
B) 2-6
C) 2-8-9
D) 2-8-8

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
17
Which radiation type was shown to be identical to N electron?
A) alpha
B) beta
C) gamma
D) None are identical to an electron.
A) alpha
B) beta
C) gamma
D) None are identical to an electron.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
18
John Dalton did his research work in which of the following countries?
A) France
B) Greece
C) Russia
D) England
A) France
B) Greece
C) Russia
D) England

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
19
Sunburn and some forms of skin cancer are caused by what kind of light?
A) infrared, IR
B) gamma,
C) microwave
D) ultraviolet, UV
A) infrared, IR
B) gamma,
C) microwave
D) ultraviolet, UV

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
20
Which subatomic particle has the incorrect charge indicated?
A) proton - positive
B) electron - negative
C) neutron - neutral
D) nucleus - neutral
A) proton - positive
B) electron - negative
C) neutron - neutral
D) nucleus - neutral

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
21
The STM, scanning tunneling microscope, shows
A) the outer boundary surface of the electrons in an atom
B) number of excited electrons in an atom
C) the charge on the nucleus
D) the mass of the nucleus
A) the outer boundary surface of the electrons in an atom
B) number of excited electrons in an atom
C) the charge on the nucleus
D) the mass of the nucleus

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
22
Who proposed the law of definite proportions?
A) Thomson
B) Rutherford
C) Dalton
D) Proust
A) Thomson
B) Rutherford
C) Dalton
D) Proust

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
23
Which element is a noble gas?
A) Ni
B) Ne
C) Si
D) B
A) Ni
B) Ne
C) Si
D) B

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
24
The majority of the elements are
A) gases.
B) nonmetals.
C) radioactive.
D) metals.
A) gases.
B) nonmetals.
C) radioactive.
D) metals.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
25
What name is given to the sum of neutrons and protons in an atom's nucleus?
A) atomic number
B) mass number
C) isotope number
D) atomic mole mass
A) atomic number
B) mass number
C) isotope number
D) atomic mole mass

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following ground state configurations matches represents an atom with 3 valence electrons?
A) 2-8-3
B) 2-1
C) 2-3-5
D) 2-8-8
A) 2-8-3
B) 2-1
C) 2-3-5
D) 2-8-8

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following ground state configuration matches Ar, argon?
A) 2-8-1
B) 2-2-6-2-6
C) 2-2-6-8
D) 2-8-8
A) 2-8-1
B) 2-2-6-2-6
C) 2-2-6-8
D) 2-8-8

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
28
What metal was used as the foil in Rutherford's famous scattering experiment?
A) tin
B) aluminum
C) gold
D) silver
A) tin
B) aluminum
C) gold
D) silver

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
29
An electric field can deflect a beam of beta, alpha and gamma rays. Which ray will be deflected the least?
A) alpha
B) beta
C) gamma
D) All are deflected by the same amount.
A) alpha
B) beta
C) gamma
D) All are deflected by the same amount.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
30
Carbon dioxide (CO2) always has the same formula. What principle does this illustrate?
A) law of electric neutrality
B) law of conservation of matter
C) law of definite proportions
D) no particular law
A) law of electric neutrality
B) law of conservation of matter
C) law of definite proportions
D) no particular law

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
31
Two atoms which have the same atomic number but different mass numbers are called
A) sisters.
B) neutrinos.
C) allotropes.
D) isotopes.
A) sisters.
B) neutrinos.
C) allotropes.
D) isotopes.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
32
What classification is given to the elements found in the A Groups of the periodic table?
A) representative or main-group
B) transition
C) lanthanide
D) actinide
A) representative or main-group
B) transition
C) lanthanide
D) actinide

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
33
The name given to the number of protons in an atom's nucleus is
A) atomic number.
B) family number.
C) electron number.
D) mass number.
A) atomic number.
B) family number.
C) electron number.
D) mass number.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
34
Which sequence lists a nonmetal, metalloid, and a transition metal, respectively?
A) Al, As, Ag
B) Co, Pb, P
C) O, Ge, Cs
D) N, Si, Fe
A) Al, As, Ag
B) Co, Pb, P
C) O, Ge, Cs
D) N, Si, Fe

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
35
What experimental evidence served as a basis of Bohr's atomic theory?
A) magnetic measurements
B) behavior of atoms at low temperatures
C) atomic mass
D) atomic spectra
A) magnetic measurements
B) behavior of atoms at low temperatures
C) atomic mass
D) atomic spectra

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
36
Which is the ground state of a hydrogen atom?
A) three electrons in the n = 1 level
B) one electron in the n = 2 level
C) one electron in the n = 1 level
D) one electron in the n = 1 level and one electron in the n = 2 level
A) three electrons in the n = 1 level
B) one electron in the n = 2 level
C) one electron in the n = 1 level
D) one electron in the n = 1 level and one electron in the n = 2 level

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
37
Which statement was not part of Dalton's atomic theory?
A) Matter is composed of indestructible particles called atoms.
B) All atoms of a given element are alike.
C) Elements and compounds are composed of definite arrays of atoms.
D) Some atoms may emit nuclear radiation.
A) Matter is composed of indestructible particles called atoms.
B) All atoms of a given element are alike.
C) Elements and compounds are composed of definite arrays of atoms.
D) Some atoms may emit nuclear radiation.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
38
The modern periodic table is based on arranging elements in the order of their
A) atomic weight.
B) atomic number.
C) mass number.
D) isotope number.
A) atomic weight.
B) atomic number.
C) mass number.
D) isotope number.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
39
Which is not a property of nonmetals?
A) malleable
B) insulator
C) gain electrons to form negative ions
D) poor conductor of electricity
A) malleable
B) insulator
C) gain electrons to form negative ions
D) poor conductor of electricity

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
40
Which element is found in Group 2 - in Period 4?
A) magnesium, Mg
B) zinc, Zn
C) calcium, Ca
D) potassium, K
A) magnesium, Mg
B) zinc, Zn
C) calcium, Ca
D) potassium, K

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
41
Group 2 elements, M, react with oxygen, O, to form oxides with the formula
A) MO.
B) M2O.
C) MO2.
D) M2O3.
A) MO.
B) M2O.
C) MO2.
D) M2O3.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
42
The reason for your answer in the previous question is that both cesium and sodium
A) have about the same atomic weight.
B) have about the same atomic number.
C) are both metals.
D) are in the same group of the periodic table.
A) have about the same atomic weight.
B) have about the same atomic number.
C) are both metals.
D) are in the same group of the periodic table.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
43
Which is not true about metals?
A) good conductors of heat
B) form positive ions
C) can be stretched or drawn into wires
D) many are liquids
A) good conductors of heat
B) form positive ions
C) can be stretched or drawn into wires
D) many are liquids

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following elements conducts electricity well?
A) arsenic
B) boron
C) sulfur
D) silver
A) arsenic
B) boron
C) sulfur
D) silver

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following is a transition element?
A) sodium
B) sulfur
C) boron
D) chromium
A) sodium
B) sulfur
C) boron
D) chromium

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
46
Why are atoms at the bottom of a group in the periodic table larger than atoms at the top of the group?
A) Larger atoms have more electrons and more occupied energy levels.
B) Larger atoms have more protons.
C) Larger atoms have more energy levels occupied by electrons.
D) Larger atoms have less screening effect by inner electrons.
A) Larger atoms have more electrons and more occupied energy levels.
B) Larger atoms have more protons.
C) Larger atoms have more energy levels occupied by electrons.
D) Larger atoms have less screening effect by inner electrons.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
47
If the compound formed by radium and bromine has the formula RaBr2, what is the formula of the compound formed by strontium (Sr) and iodine (I)?
A) SrI
B) Sr2I
C) SrI2
D) No correlation exists between radium and strontium.
A) SrI
B) Sr2I
C) SrI2
D) No correlation exists between radium and strontium.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
48
The periodic law of the elements states that the properties of the elements are a periodic function of
A) atomic weights.
B) atomic numbers.
C) both atomic weights and atomic numbers.
D) neither atomic weights nor atomic numbers.
A) atomic weights.
B) atomic numbers.
C) both atomic weights and atomic numbers.
D) neither atomic weights nor atomic numbers.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
49
Which element would most likely have the Lewis dot symbol shown below? 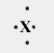
A) oxygen
B) nitrogen
C) carbon
D) fluorine
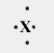
A) oxygen
B) nitrogen
C) carbon
D) fluorine

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
50
What element of the following is a metal that forms +1 ions?
A) sodium (Na)
B) aluminum (Al)
C) calcium (Ca)
D) nitrogen (N)
A) sodium (Na)
B) aluminum (Al)
C) calcium (Ca)
D) nitrogen (N)

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
51
Who was the Russian who was a pioneer in the development of the periodic law?
A) Meyerovick
B) Mendeleev
C) Dobereiner
D) Newlands
A) Meyerovick
B) Mendeleev
C) Dobereiner
D) Newlands

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
52
Which atom in the following series is the largest?
A) K
B) Rb
C) Cs
D) Na
A) K
B) Rb
C) Cs
D) Na

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following is an element in Group 14?
A) C
B) Cu
C) Cs
D) Cl
A) C
B) Cu
C) Cs
D) Cl

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
54
How many electrons in the valence shell of a calcium atom?
A) 20
B) 8
C) 2
D) 1
A) 20
B) 8
C) 2
D) 1

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following halogens has the smallest atomic radius?
A) iodine
B) fluorine
C) bromine
D) chlorine
A) iodine
B) fluorine
C) bromine
D) chlorine

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following elements is a metal?
A) Cl
B) Na
C) Ar
D) S
A) Cl
B) Na
C) Ar
D) S

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
57
If an element in a group in the periodic table has a combining power (valence) of two, another element in the same group likely has a combining power of ____.
A) two
B) one or three
C) an unpredictable number
D) four or five, depending on the element's position in the group
A) two
B) one or three
C) an unpredictable number
D) four or five, depending on the element's position in the group

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
58
Which group of elements consists of all alkaline earth elements?
A) Mg, Cl, Na, As
B) Ca, Mg, Ba, Ra
C) Ba, Al, Na, As
D) Na, K, Li, Rb
A) Mg, Cl, Na, As
B) Ca, Mg, Ba, Ra
C) Ba, Al, Na, As
D) Na, K, Li, Rb

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
59
In the following set, which atom is the smallest?
A) Ar
B) Mg
C) Cl
D) Si
A) Ar
B) Mg
C) Cl
D) Si

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
60
Sodium has chemical properties most like
A) cesium, Cs.
B) magnesium, Mg.
C) chlorine, Cl.
D) mercury, Hg.
A) cesium, Cs.
B) magnesium, Mg.
C) chlorine, Cl.
D) mercury, Hg.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
61
Which subatomic particles are found in the nucleus of 32P atom?
A) 15 protons, 15 electrons, 17 neutrons
B) 15 protons, 15 electrons, 32 neutrons
C) 15 protons, 32 neutrons
D) 15 protons, 17 neutrons
A) 15 protons, 15 electrons, 17 neutrons
B) 15 protons, 15 electrons, 32 neutrons
C) 15 protons, 32 neutrons
D) 15 protons, 17 neutrons

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
62
Which law states that matter is neither lost nor gained during a chemical reaction?
A) law of multiple proportions
B) law of definite proportions
C) law of chemical reactions
D) law of conservation of mass
A) law of multiple proportions
B) law of definite proportions
C) law of chemical reactions
D) law of conservation of mass

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following is a property of metals?
A) malleable
B) conductor
C) lose electrons to form positive ions
D) all of the above
A) malleable
B) conductor
C) lose electrons to form positive ions
D) all of the above

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
64
The fourth energy level can hold a maximum of how many electrons?
A) 2
B) 8
C) 18
D) 32
A) 2
B) 8
C) 18
D) 32

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
65
What is the ground state configuration for an argon atom?
A) 1s22s22p63s23p6
B) 1s22s22p63s23p8
C) 1s22s22p6
D) 1s22s22p63s2
A) 1s22s22p63s23p6
B) 1s22s22p63s23p8
C) 1s22s22p6
D) 1s22s22p63s2

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
66
An element with two valence (bonding, or outer shell) electrons is
A) Mg.
B) Na.
C) Cl.
D) Al.
A) Mg.
B) Na.
C) Cl.
D) Al.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
67
According to the periodic table all the elements in Group 2 are
A) noble gases.
B) metals.
C) metalloids.
D) nonmetals.
A) noble gases.
B) metals.
C) metalloids.
D) nonmetals.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following elements has 8 valence electrons?
A) Be
B) F
C) Na
D) Ne
A) Be
B) F
C) Na
D) Ne

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following elements has five valence electrons?
A) Be
B) F
C) P
D) Xe
A) Be
B) F
C) P
D) Xe

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following ground state configurations matches represents an atom with 7 valence electrons?
A) 1s22s22p63s23p6
B) 1s22s22p63s23p4
C) 1s22s22p63s2
D) 1s22s22p63s23p5
A) 1s22s22p63s23p6
B) 1s22s22p63s23p4
C) 1s22s22p63s2
D) 1s22s22p63s23p5

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
71
Which type of electromagnetic radiation has the least amount of energy per photon?
A) infrared
B) gamma
C) radio waves
D) ultraviolet
A) infrared
B) gamma
C) radio waves
D) ultraviolet

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
72
The periodic table is helpful in all the following endeavors but one. Which is the exception?
A) predicting the number of isotopes of elements
B) predicting chemical reactivity of elements
C) predicting physical properties of elements
D) predicting formula of compounds
A) predicting the number of isotopes of elements
B) predicting chemical reactivity of elements
C) predicting physical properties of elements
D) predicting formula of compounds

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
73
What is the symbol for a neutral atom with 35 protons, 35 electrons, and 46 neutrons?
A) Se
B) Br
C) Cl
D) Ti
A) Se
B) Br
C) Cl
D) Ti

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
74
What general electronic arrangement is characteristic of chemical inactivity?
A) a total of eight electrons per atom
B) filled s and p orbitals
C) all electrons paired
D) 2-8-8
A) a total of eight electrons per atom
B) filled s and p orbitals
C) all electrons paired
D) 2-8-8

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following elements is found in Group 16 and in Period 3?
A) phosphorus, P
B) chromium, Cr
C) sulfur, S
D) oxygen, O
A) phosphorus, P
B) chromium, Cr
C) sulfur, S
D) oxygen, O

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
76
An element with electronic structure of 2-8-3 is in which of the following groups of the periodic table?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following elements is a nonmetal?
A) potassium
B) copper
C) sulfur
D) lithium
A) potassium
B) copper
C) sulfur
D) lithium

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
78
An element with electronic structure of 2-8-8 is found in which of the following groups of the periodic table?
A) 2
B) 4
C) 5
D) 8
A) 2
B) 4
C) 5
D) 8

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
79
Which element has the following ground state electron configuration 1s22s22p63s23p64s1?
A) Ar
B) Na
C) K
D) Cl
A) Ar
B) Na
C) K
D) Cl

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following reacts most violently with water?
A) Ne, neon
B) Na, sodium
C) Li, lithium
D) K, potassium
A) Ne, neon
B) Na, sodium
C) Li, lithium
D) K, potassium

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck


