Exam 3: Atoms and the Periodic Table
Exam 1: Living in a World of Chemistry21 Questions
Exam 2: The Chemical View of Matter59 Questions
Exam 3: Atoms and the Periodic Table112 Questions
Exam 4: The Air We Breathe48 Questions
Exam 5: Chemical Bonding and States of Matter89 Questions
Exam 6: Carbon Dioxide and the Greenhouse Effect31 Questions
Exam 7: Chlorofluorocarbons and the Ozone Layer29 Questions
Exam 8: Chemical Reactivity: Chemicals in Action54 Questions
Exam 9: Acid-Base Reactions68 Questions
Exam 10: Oxidation-Reduction Reactions47 Questions
Exam 11: Water, Water Everywhere but Not a Drop to Drink50 Questions
Exam 12: Energy and Hydrocarbons57 Questions
Exam 13: Nuclear Changes and Nuclear Power73 Questions
Exam 14: Organic Chemicals and Polymers61 Questions
Exam 15: The Chemistry of Life86 Questions
Exam 16: Nutrition: the Basis of Healthy Living61 Questions
Exam 17: Chemistry and Medicine86 Questions
Exam 18: The Chemistry of Useful Materials65 Questions
Exam 19: Feeding the World54 Questions
Select questions type
What classification is given to the elements found in the A Groups of the periodic table?
Free
(Multiple Choice)
4.8/5  (32)
(32)
Correct Answer:
A
Which subatomic particle has the incorrect charge indicated?
Free
(Multiple Choice)
4.9/5  (41)
(41)
Correct Answer:
D
As the wavelength of electron magnetic radiation increases, the energy of the wave ____________.
Free
(Short Answer)
5.0/5  (33)
(33)
Correct Answer:
decreases
What element of the following is a metal that forms +1 ions?
(Multiple Choice)
4.9/5  (26)
(26)
What metal was used as the foil in Rutherford's famous scattering experiment?
(Multiple Choice)
4.8/5  (47)
(47)
Who was the first person to propose a consistent modern atomic theory?
(Multiple Choice)
4.9/5  (39)
(39)
MATCHING
Consider the image below which represents an individual block on the periodic table.  Answer the following questions with the appropriate letter from the list given below.
-What does the "X" represent?
Answer the following questions with the appropriate letter from the list given below.
-What does the "X" represent?
(Multiple Choice)
4.8/5  (35)
(35)
How many protons, neutrons, and electrons are in the following isotope, respectively? 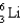
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following halogens has the smallest atomic radius?
(Multiple Choice)
4.8/5  (39)
(39)
If an element in a group in the periodic table has a combining power (valence) of two, another element in the same group likely has a combining power of ____.
(Multiple Choice)
4.7/5  (40)
(40)
MATCHING
Consider the image below which represents an individual block on the periodic table.  Answer the following questions with the appropriate letter from the list given below.
-What does the "22" represent?
Answer the following questions with the appropriate letter from the list given below.
-What does the "22" represent?
(Multiple Choice)
4.8/5  (32)
(32)
What name is given to the sum of neutrons and protons in an atom's nucleus?
(Multiple Choice)
4.9/5  (36)
(36)
Use the Lewis symbol shown below to answer the following questions.  -An element having this Lewis dot symbol would be classified as a __________.
-An element having this Lewis dot symbol would be classified as a __________.
(Short Answer)
4.8/5  (31)
(31)
An element with electronic structure of 2-8-8 is found in which of the following groups of the periodic table?
(Multiple Choice)
4.8/5  (39)
(39)
The elements that are good conductors of heat and electricity are the metals.
(True/False)
4.9/5  (39)
(39)
Showing 1 - 20 of 112
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)