Deck 8: Electron Configuration and Chemical Periodicity
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/85
Play
Full screen (f)
Deck 8: Electron Configuration and Chemical Periodicity
1
Select the correct set of quantum numbers (n, l, ml, ms) for the highest energy electron in the ground state of potassium, K.
A) 4, 1, -1,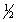
B) 4, 1, 0,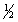
C) 4, 0, 1,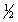
D) 4, 0, 0,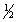
E) 4, 1, 1,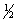
A) 4, 1, -1,
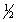
B) 4, 1, 0,
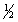
C) 4, 0, 1,
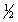
D) 4, 0, 0,
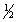
E) 4, 1, 1,
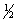
4, 0, 0, 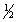
2
Select the correct electron configuration for Te (Z = 52).
A) [Kr]5s25p64d8
B) [Kr]5s25d105p4
C) [Kr]5s24d105p6
D) [Kr]5s24f14
E) [Kr]5s24d105p4
A) [Kr]5s25p64d8
B) [Kr]5s25d105p4
C) [Kr]5s24d105p6
D) [Kr]5s24f14
E) [Kr]5s24d105p4
[Kr]5s24d105p4
3
Which one of the following statements about orbital energies is incorrect?
A) In the hydrogen atom, the energy of an orbital depends only on the value of the quantum number n.
B) In many-electron atoms the energy of an orbital depends on both n and l.
C) Inner electrons shield outer electrons more effectively than do electrons in the same orbital.
D) The splitting of sublevels in many-electron atoms is explained in terms of the penetration effect.
E) The energy of a given orbital increases as the nuclear charge Z increases.
A) In the hydrogen atom, the energy of an orbital depends only on the value of the quantum number n.
B) In many-electron atoms the energy of an orbital depends on both n and l.
C) Inner electrons shield outer electrons more effectively than do electrons in the same orbital.
D) The splitting of sublevels in many-electron atoms is explained in terms of the penetration effect.
E) The energy of a given orbital increases as the nuclear charge Z increases.
The energy of a given orbital increases as the nuclear charge Z increases.
4
Which one of the following statements about atomic structure and quantum numbers is incorrect?
A) In a given atom, the maximum number of electrons having principal quantum number n = 3, is 18.
B) The number of orbitals in a given f subshell is 7.
C) For n = 4, the largest possible value of l is 3.
D) For n = 4, the largest possible value of ml is 2.
E) The following set of quantum numbers for a single orbital is not allowed: n = 3, l = 1, ml = -2.
A) In a given atom, the maximum number of electrons having principal quantum number n = 3, is 18.
B) The number of orbitals in a given f subshell is 7.
C) For n = 4, the largest possible value of l is 3.
D) For n = 4, the largest possible value of ml is 2.
E) The following set of quantum numbers for a single orbital is not allowed: n = 3, l = 1, ml = -2.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
5
Select the correct electron configuration for sulfur (Z = 16).
A) 1s21p62s22p6
B) 1s22s22p83s23p4
C) 1s22s22p83s23p2
D) 1s22s22p63s23p4
E) 1s22s22p63s23d4
A) 1s21p62s22p6
B) 1s22s22p83s23p4
C) 1s22s22p83s23p2
D) 1s22s22p63s23p4
E) 1s22s22p63s23d4

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
6
The effective nuclear charge for an atom is less than the actual nuclear charge due to
A) shielding.
B) penetration.
C) paramagnetism.
D) electron-pair repulsion.
E) relativity.
A) shielding.
B) penetration.
C) paramagnetism.
D) electron-pair repulsion.
E) relativity.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
7
Select the correct electron configuration for Cu (Z = 29).
A) [Ar]4s23d9
B) [Ar]4s13d10
C) [Ar]4s24p63d3
D) [Ar]4s24d9
E) [Ar]5s24d9
A) [Ar]4s23d9
B) [Ar]4s13d10
C) [Ar]4s24p63d3
D) [Ar]4s24d9
E) [Ar]5s24d9

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
8
How many valence electrons are there in an atom with the electron configuration [noble gas]ns2(n - 1)d10np3?
A) 2
B) 3
C) 5
D) 10
E) 15
A) 2
B) 3
C) 5
D) 10
E) 15

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
9
Select the correct set of quantum numbers (n, l, ml, ms) for the first electron removed in the formation of a cation for strontium, Sr.
A) 5, 1 , 0, -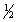
B) 5, 1, 0,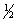
C) 5, 0, 1,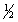
D) 5, 1, 1,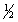
E) 5, 0, 0, -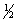
A) 5, 1 , 0, -
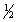
B) 5, 1, 0,
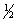
C) 5, 0, 1,
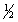
D) 5, 1, 1,
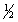
E) 5, 0, 0, -

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
10
Energy states of atoms containing more than one electron arise from nucleus-electron and electron-electron interactions. Which of the following statements correctly describes these effects?
A) Larger nuclear charge lowers energy, more electrons in an orbital lowers energy.
B) Larger nuclear charge lowers energy, more electrons in an orbital increases energy.
C) Smaller nuclear charge lowers energy, more electrons in an orbital lowers energy.
D) Smaller nuclear charge lowers energy, more electrons in an orbital increases energy.
E) None of the above statements is generally correct.
A) Larger nuclear charge lowers energy, more electrons in an orbital lowers energy.
B) Larger nuclear charge lowers energy, more electrons in an orbital increases energy.
C) Smaller nuclear charge lowers energy, more electrons in an orbital lowers energy.
D) Smaller nuclear charge lowers energy, more electrons in an orbital increases energy.
E) None of the above statements is generally correct.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
11
The electronic structure 1s22s22p63s23p64s23d8 refers to the ground state of
A) Kr.
B) Ni.
C) Fe.
D) Pd.
E) none of the above.
A) Kr.
B) Ni.
C) Fe.
D) Pd.
E) none of the above.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following electron configurations is impossible?
A) 1s22s22p63s23p4
B) 1s22s22p53s13p4
C) 1s22s22p63s23p63d104s2
D) 1s22s22p63s33p4
E) 1s12s22p63s23p4
A) 1s22s22p63s23p4
B) 1s22s22p53s13p4
C) 1s22s22p63s23p63d104s2
D) 1s22s22p63s33p4
E) 1s12s22p63s23p4

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
13
Select the correct set of quantum numbers (n, l, ml, ms) for the highest energy electron in the ground state of tin, Sn.
A) 5, 2, -1,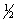
B) 5, 2, 0,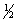
C) 5, 1, 2,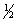
D) 5, 1, 0,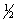
E) 5, 2, 1,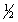
A) 5, 2, -1,
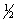
B) 5, 2, 0,
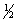
C) 5, 1, 2,
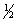
D) 5, 1, 0,
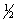
E) 5, 2, 1,

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following sublevels is filled last? 3d, 4s, 4p, 4d, 5s
A) 3d
B) 4s
C) 4p
D) 4d
E) 5s
A) 3d
B) 4s
C) 4p
D) 4d
E) 5s

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
15
"Each electron in an atom must have its own unique set of quantum numbers" is a statement of
A) the aufbau principle.
B) the Pauli exclusion principle.
C) Hund's rule.
D) the periodic law.
E) Heisenberg's principle.
A) the aufbau principle.
B) the Pauli exclusion principle.
C) Hund's rule.
D) the periodic law.
E) Heisenberg's principle.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
16
An atom of element number 33 (As) is in its ground electronic state. Which one of the following sets quantum numbers could not apply to any of its electrons?
A) n = 2 l = 1 ml = -1 ms = +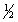
B) n = 3 l = 0 ml = 0 ms = -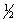
C) n = 3 l = 2 ml = -2 ms = -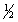
D) n = 4 l = 0 ml = 0 ms = -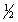
E) n = 4 l = 2 ml = 1 ms = +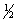
A) n = 2 l = 1 ml = -1 ms = +
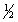
B) n = 3 l = 0 ml = 0 ms = -
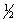
C) n = 3 l = 2 ml = -2 ms = -
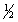
D) n = 4 l = 0 ml = 0 ms = -
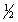
E) n = 4 l = 2 ml = 1 ms = +

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
17
In the ground state of an atom of silver (Ag), how many electrons will there be with the quantum number l = 1? (The n, ml, and ms quantum numbers may have any appropriate values.)
A) 9
B) 12
C) 18
D) 24
E) 36
A) 9
B) 12
C) 18
D) 24
E) 36

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
18
"Electrons added to atomic orbitals of the same energy will remain unpaired with parallel spins until the subshell is more than half-filled" is a statement of
A) the aufbau principle.
B) Hund's rule.
C) the Pauli exclusion principle.
D) the periodic law.
E) the singularity rule.
A) the aufbau principle.
B) Hund's rule.
C) the Pauli exclusion principle.
D) the periodic law.
E) the singularity rule.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
19
In a single atom, what is the maximum number of electrons which can have quantum number n = 4?
A) 16
B) 18
C) 32
D) 36
E) none of the above
A) 16
B) 18
C) 32
D) 36
E) none of the above

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
20
In many-electron atoms, which quantum numbers specify the energy of an electron?
A) n and l
B) n and ml
C) l and ml
D) n and ms
E) n, l, and ml
A) n and l
B) n and ml
C) l and ml
D) n and ms
E) n, l, and ml

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following electron configurations represents the ground state of an element?
A) [Ne]3s13p1
B) [He]2s12p3
C) [Ne]3s23p23d1
D) [Ne]3s23p33d1
E) [Ne]3s23p3
A) [Ne]3s13p1
B) [He]2s12p3
C) [Ne]3s23p23d1
D) [Ne]3s23p33d1
E) [Ne]3s23p3

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
22
In the electron configuration [Ar]4s23d104p4, which are valence electrons?
A) all of the electrons after the [Ar]
B) only the 4s2 electrons
C) only the 3d10 electrons
D) only the 4p4 electrons
E) both the 4s2 and the 4p4 electrons
A) all of the electrons after the [Ar]
B) only the 4s2 electrons
C) only the 3d10 electrons
D) only the 4p4 electrons
E) both the 4s2 and the 4p4 electrons

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
23
When comparing the successive ionization energies of an element, an unusually big increase in ionization energy is seen when
A) the first valence electron is removed.
B) the second valence electron is removed.
C) the eighth electron of is removed.
D) the first core electron is removed.
E) the last valence electron is removed.
A) the first valence electron is removed.
B) the second valence electron is removed.
C) the eighth electron of is removed.
D) the first core electron is removed.
E) the last valence electron is removed.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following elements has the smallest atomic radius?
A) Li
B) Ne
C) Rb
D) Sr
E) Xe
A) Li
B) Ne
C) Rb
D) Sr
E) Xe

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following elements has the greatest atomic radius?
A) Li
B) Ne
C) Rb
D) Sr
E) Xe
A) Li
B) Ne
C) Rb
D) Sr
E) Xe

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following elements has the smallest atomic size?
A) Na
B) Ar
C) K
D) Ca
E) Kr
A) Na
B) Ar
C) K
D) Ca
E) Kr

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following elements has the largest atomic size?
A) S
B) Ca
C) Ba
D) Po
E) Rn
A) S
B) Ca
C) Ba
D) Po
E) Rn

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
28
Elements with the highest first ionization energies are found in the ___________ region of the periodic table.
A) lower left
B) upper left
C) center
D) lower right
E) upper right
A) lower left
B) upper left
C) center
D) lower right
E) upper right

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
29
Which one of the following equations correctly represents the process relating to the ionization energy of X?
A) X(s) X+(g) + e-
B) X2(g) X+(g) + X-(g)
C) X(g) + e- X-(g)
D) X-(g) X(g) + e-
E) X(g) X+(g) + e-
A) X(s) X+(g) + e-
B) X2(g) X+(g) + X-(g)
C) X(g) + e- X-(g)
D) X-(g) X(g) + e-
E) X(g) X+(g) + e-

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following fourth-period elements has the smallest atomic radius?
A) K
B) Ti
C) Cu
D) Ge
E) Kr
A) K
B) Ti
C) Cu
D) Ge
E) Kr

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following electron configurations is not possible for an atom in an excited state?
A) 1s22s22p63s23p63d104s14p1
B) 1s22s22p63s13p5
C) 1s22s22p63s23p23d2
D) 1s22s22p63s23p63d104s2
E) 1s22s22p63s23p63d104s14p3
A) 1s22s22p63s23p63d104s14p1
B) 1s22s22p63s13p5
C) 1s22s22p63s23p23d2
D) 1s22s22p63s23p63d104s2
E) 1s22s22p63s23p63d104s14p3

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
32
Identify the element of Period 2 which has the following successive ionization energies, in kJ/mol. IE1, 1314 IE2, 3389 IE3, 5298 IE4, 7471
IE5, 10992 IE6, 13329 IE7, 71345 IE8, 84087
A) Li
B) B
C) O
D) Ne
E) none of the above
IE5, 10992 IE6, 13329 IE7, 71345 IE8, 84087
A) Li
B) B
C) O
D) Ne
E) none of the above

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following elements has the largest second ionization energy (IE2)?
A) Li
B) B
C) O
D) F
E) Na
A) Li
B) B
C) O
D) F
E) Na

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following sets of elements have the [noble gas]ns2np2 valence electron configuration?
A) O, S, Se, Te, Po
B) N, P, As, Sb, Bi
C) F, Cl, Br, I, At
D) C, Si, Ge, Sn, Pb
E) Ti, Zr, Hf
A) O, S, Se, Te, Po
B) N, P, As, Sb, Bi
C) F, Cl, Br, I, At
D) C, Si, Ge, Sn, Pb
E) Ti, Zr, Hf

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
35
Select the element with the most negative electron affinity (i.e., accepts an electron most readily).
A) H
B) Li
C) C
D) F
E) Ne
A) H
B) Li
C) C
D) F
E) Ne

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
36
Which one of the following equations correctly represents the process involved in the electron affinity of X?
A) X(g) X+(g) + e-
B) X+(g) X+(aq)
C) X+(g) + e- X(g)
D) X(g) + e- X-(g)
E) X+(g) + Y-(g) XY(s)
A) X(g) X+(g) + e-
B) X+(g) X+(aq)
C) X+(g) + e- X(g)
D) X(g) + e- X-(g)
E) X+(g) + Y-(g) XY(s)

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
37
How many valence electrons are there in an atom with the electron configuration [noble gas]ns2(n - 1)d8?
A) 2
B) 6
C) 8
D) 10
E) none of the above
A) 2
B) 6
C) 8
D) 10
E) none of the above

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following elements has the smallest first ionization energy?
A) Rb
B) Mg
C) I
D) As
E) F
A) Rb
B) Mg
C) I
D) As
E) F

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following elements has the largest first ionization energy?
A) Na
B) Cl
C) Ca
D) Te
E) Br
A) Na
B) Cl
C) Ca
D) Te
E) Br

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following electron configurations is correct for the excited state of an element?
A) [He]2s22p5
B) [Ne]3s23p1
C) [Ar]4s14p1
D) [Kr]5s24d7
E) [He]1p1
A) [He]2s22p5
B) [Ne]3s23p1
C) [Ar]4s14p1
D) [Kr]5s24d7
E) [He]1p1

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
41
Select the diamagnetic ion.
A) Cu2+
B) Ni2+
C) Cr3+
D) Sc3+
E) Cr2+
A) Cu2+
B) Ni2+
C) Cr3+
D) Sc3+
E) Cr2+

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
42
Select the most acidic compound from the following:
A) SO2
B) Al2O3
C) CaO
D) PbO
E) H2O
A) SO2
B) Al2O3
C) CaO
D) PbO
E) H2O

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following has the most negative electron affinity?
A) H
B) Li
C) Na
D) K
E) Rb
A) H
B) Li
C) Na
D) K
E) Rb

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
44
Consider the set of isoelectronic atoms and ions A2-, B-, C, D+, and E2+. Which arrangement of relative radii is correct?
A) A2- > B- > C > D+ > E2+
B) E2+ > D+ > C > B- > A2-
C) A2- > B- > C < D+ < E2+
D) A2- < B- < C > D+ > E2+
E) None of the above is correct.
A) A2- > B- > C > D+ > E2+
B) E2+ > D+ > C > B- > A2-
C) A2- > B- > C < D+ < E2+
D) A2- < B- < C > D+ > E2+
E) None of the above is correct.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
45
In a single atom, write down the maximum possible number of electrons
a. with n = 3
b. with the designation 4dxy
c. with n = 5, l = 3, ms = +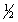
a. with n = 3
b. with the designation 4dxy
c. with n = 5, l = 3, ms = +

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
46
Elements with _______________ first ionization energies and ___________ electron affinities generally form anions.
A) low, very negative
B) high, positive or slightly negative
C) low, positive or slightly negative
D) high, very negative
E) None of the above is generally correct.
A) low, very negative
B) high, positive or slightly negative
C) low, positive or slightly negative
D) high, very negative
E) None of the above is generally correct.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
47
The most basic oxides are formed from elements found in the __________________ region of the periodic table.
A) upper right
B) upper left
C) center
D) lower right
E) lower left
A) upper right
B) upper left
C) center
D) lower right
E) lower left

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
48
Write down the maximum number of electrons in an atom which can have
a. quantum number n = 4.
b. orbital designation 3d.
c. orbital designation 2pz.
a. quantum number n = 4.
b. orbital designation 3d.
c. orbital designation 2pz.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
49
Metallic behavior is generally associated with
A) elements with very negative electron affinities.
B) elements with low ionization energies.
C) elements with small atomic radii.
D) elements with unpaired electrons.
E) elements with partially filled p orbitals.
A) elements with very negative electron affinities.
B) elements with low ionization energies.
C) elements with small atomic radii.
D) elements with unpaired electrons.
E) elements with partially filled p orbitals.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
50
Select the paramagnetic ion.
A) Cu+
B) Ag+
C) Fe3+
D) Cd2+
E) Ca2+
A) Cu+
B) Ag+
C) Fe3+
D) Cd2+
E) Ca2+

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following atoms will be diamagnetic?
A) Cr
B) Ru
C) Fe
D) Pt
E) Cd
A) Cr
B) Ru
C) Fe
D) Pt
E) Cd

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following ions will be most likely to form when selenium ionizes?
A) Se6+
B) Se4+
C) Se2+
D) Se2-
E) Se4-
A) Se6+
B) Se4+
C) Se2+
D) Se2-
E) Se4-

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
53
Select the most basic compound from the following:
A) Bi2O3
B) SiO2
C) Cs2O
D) Na2O
E) H2O
A) Bi2O3
B) SiO2
C) Cs2O
D) Na2O
E) H2O

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
54
Select the element with the least metallic character.
A) Sn
B) Sr
C) Tl
D) Ge
E) Ga
A) Sn
B) Sr
C) Tl
D) Ge
E) Ga

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
55
Select the element with the greatest metallic character.
A) Li
B) Ca
C) Al
D) Pb
E) Cs
A) Li
B) Ca
C) Al
D) Pb
E) Cs

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
56
Elements with ________________ first ionization energies and ___________ electron affinities generally form cations.
A) low, very negative
B) high, positive or slightly negative
C) low, positive or slightly negative
D) high, very negative
E) None of the above is generally correct.
A) low, very negative
B) high, positive or slightly negative
C) low, positive or slightly negative
D) high, very negative
E) None of the above is generally correct.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
57
What is the correct order of decreasing size of the following ions?
A) P3- > Cl- > K+ > Ca2+
B) Ca2+ > K+ > Cl- > P3-
C) K+ > Cl- > Ca2+ > P3-
D) K+ > Cl- > P3- > Ca2+
E) None of the above is correct.
A) P3- > Cl- > K+ > Ca2+
B) Ca2+ > K+ > Cl- > P3-
C) K+ > Cl- > Ca2+ > P3-
D) K+ > Cl- > P3- > Ca2+
E) None of the above is correct.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following elements is paramagnetic?
A) Kr
B) Zn
C) Sr
D) V
E) Ar
A) Kr
B) Zn
C) Sr
D) V
E) Ar

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
59
The most acidic oxides are formed from elements found in the _________________ region of the periodic table.
A) upper right
B) upper left
C) center
D) lower right
E) lower left
A) upper right
B) upper left
C) center
D) lower right
E) lower left

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following elements will form a cation with a +2 charge?
A) Si
B) Sr
C) Ga
D) Cs
E) S
A) Si
B) Sr
C) Ga
D) Cs
E) S

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
61
Define what is meant by electron affinity, and write a balanced chemical equation to represent the relevant process for element Y.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
62
In neutral atoms, the 3d orbitals have higher energy than the 4s orbitals.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
63
Hund's rule is used to predict the electron configuration of atoms in excited states.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
64
Elements in which the outermost electron has the same principal quantum number n, show similar chemical properties.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
65
Describe how the ionization energies of main group elements vary across a period in the periodic table, using a diagram if necessary.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
66
Briefly describe how the atomic radii and ionization energies of group 1A(1) elements compare with those of group 8A(18). Also, explain why the values of these properties are so different between these two groups.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
67
Elements in the same period of the periodic table have similar valence shell electron configurations.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
68
Write down the full electron configuration for ground state atoms of
a. element number 12.
b. element number 23.
c. element number 32.
a. element number 12.
b. element number 23.
c. element number 32.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
69
Atomic size decreases across a period due to an increase in the effective nuclear charge, Zeff.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
70
In Mendeleev's version of the periodic table, the elements were arranged in order of increasing atomic number.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
71
Bigger atoms generally have smaller ionization energies.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
72
State Hund's rule, and show how it applies to the ground state of phosphorus atoms.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
73
Write down the full electron configuration for ground state atoms of
a. S (element 16).
b. Pd (element 46).
a. S (element 16).
b. Pd (element 46).

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
74
Moseley's measurements of nuclear charges of the elements provided the basis for arranging the elements of the periodic table in order of increasing atomic number.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
75
The maximum number of electrons in an atom with the same value of n is 2n2.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
76
According to the exclusion principle, two is the maximum number of electrons in an atom which can share the same four quantum numbers.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
77
Define what is meant by ionization energy, and write a balanced chemical equation to represent the relevant process for element X.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
78
First ionization energies of neutral atoms may be positive or negative.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
79
Describe how the atomic radii of main group elements vary with position in the periodic table.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
80
The difference in energies between the 1s and 2s orbitals is due to the penetration effect.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck


