Exam 8: Electron Configuration and Chemical Periodicity
Exam 1: Keys to the Study of Chemistry79 Questions
Exam 2: The Components of Matter105 Questions
Exam 3: Stoichiometry of Formulas and Equations87 Questions
Exam 4: The Major Classes of Chemical Reactions124 Questions
Exam 5: Gases and the Kinetic-Molecular Theory115 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change85 Questions
Exam 7: Quantum Theory and Atomic Structure83 Questions
Exam 8: Electron Configuration and Chemical Periodicity85 Questions
Exam 9: Models of Chemical Bonding74 Questions
Exam 10: The Shapes of Molecules109 Questions
Exam 11: Theories of Covalent Bonding58 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes111 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids105 Questions
Exam 14: Periodic Patterns in the Main Group Elements: Bonding, Structure, and Reactivity118 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon118 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions88 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions104 Questions
Exam 18: Acid-Base Equilibria103 Questions
Exam 19: Ionic Equilibria in Aqueous Systems119 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions94 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry57 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications94 Questions
Select questions type
Elements with the highest first ionization energies are found in the ___________ region of the periodic table.
Free
(Multiple Choice)
4.8/5  (46)
(46)
Correct Answer:
E
Write down the full electron configuration for ground state atoms of
a. element number 12.
b. element number 23.
c. element number 32.
Free
(Essay)
4.9/5  (41)
(41)
Correct Answer:
a. 1s22s22p63s2
b. 1s22s22p63s23p64s23d3
c. 1s22s22p63s23p64s23d104p2
Which of the following has the most negative electron affinity?
Free
(Multiple Choice)
4.7/5  (35)
(35)
Correct Answer:
A
According to the exclusion principle, two is the maximum number of electrons in an atom which can share the same four quantum numbers.
(True/False)
4.8/5  (32)
(32)
Which of the following elements has the largest first ionization energy?
(Multiple Choice)
4.9/5  (37)
(37)
In neutral atoms, the 3d orbitals have higher energy than the 4s orbitals.
(True/False)
4.7/5  (20)
(20)
In the ground state of an atom of silver (Ag), how many electrons will there be with the quantum number l = 1? (The n, ml, and ms quantum numbers may have any appropriate values.)
(Multiple Choice)
4.9/5  (33)
(33)
Elements in the same period of the periodic table have similar valence shell electron configurations.
(True/False)
4.8/5  (34)
(34)
Which one of the following statements about orbital energies is incorrect?
(Multiple Choice)
4.9/5  (31)
(31)
Which of the following sets of elements have the [noble gas]ns2np2 valence electron configuration?
(Multiple Choice)
4.7/5  (37)
(37)
"Electrons added to atomic orbitals of the same energy will remain unpaired with parallel spins until the subshell is more than half-filled" is a statement of
(Multiple Choice)
4.7/5  (33)
(33)
The most basic oxides are formed from elements found in the __________________ region of the periodic table.
(Multiple Choice)
4.8/5  (33)
(33)
Atomic size decreases across a period due to an increase in the effective nuclear charge, Zeff.
(True/False)
4.8/5  (25)
(25)
Which of the following electron configurations is impossible?
(Multiple Choice)
4.7/5  (33)
(33)
Which of the following elements has the largest atomic size?
(Multiple Choice)
4.7/5  (38)
(38)
Moseley's measurements of nuclear charges of the elements provided the basis for arranging the elements of the periodic table in order of increasing atomic number.
(True/False)
4.9/5  (31)
(31)
In a single atom, write down the maximum possible number of electrons
a. with n = 3
b. with the designation 4dxy
c. with n = 5, l = 3, ms = + 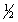
(Essay)
4.8/5  (31)
(31)
Showing 1 - 20 of 85
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)