Deck 23: The Transition Elements and Their Coordination Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/92
Play
Full screen (f)
Deck 23: The Transition Elements and Their Coordination Compounds
1
What is the highest possible oxidation state for molybdenum, Mo?
A) +2
B) +4
C) +6
D) +8
E) none of the above
A) +2
B) +4
C) +6
D) +8
E) none of the above
+6
2
Which of the following atoms has the biggest radius?
A) Ti
B) Cr
C) Fe
D) Ni
E) Zn
A) Ti
B) Cr
C) Fe
D) Ni
E) Zn
Ti
3
A certain transition element has the stable oxidation states of +2, +3, +4, +5, and +6. In which state will the element be most likely to form an ionic bond with chlorine?
A) +2
B) +3
C) +4
D) +5
E) +6
A) +2
B) +3
C) +4
D) +5
E) +6
+2
4
The most common oxidation state for ions of the inner transition elements is
A) +2.
B) +3.
C) +4.
D) +5.
E) +7.
A) +2.
B) +3.
C) +4.
D) +5.
E) +7.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
5
What is the highest possible oxidation state for palladium, Pd?
A) +1
B) +2
C) +3
D) +4
E) +6
A) +1
B) +2
C) +3
D) +4
E) +6

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following ions is least likely to form colored compounds?
A) Mn2+
B) Cr5+
C) Sc3+
D) Fe3+
E) Co2+
A) Mn2+
B) Cr5+
C) Sc3+
D) Fe3+
E) Co2+

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
7
The most common oxidation state for ions of the transition elements is
A) +2.
B) +3.
C) +4.
D) +5.
E) +6.
A) +2.
B) +3.
C) +4.
D) +5.
E) +6.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following elements has the ground state electron configuration, [Xe]4f145d106s1?
A) Hg
B) Ag
C) Hf
D) Au
E) Th
A) Hg
B) Ag
C) Hf
D) Au
E) Th

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following ions is most likely to form colored compounds?
A) Sc3+
B) Cu+
C) Zn2+
D) Cr3+
E) Ca2+
A) Sc3+
B) Cu+
C) Zn2+
D) Cr3+
E) Ca2+

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
10
A certain transition element has the stable oxidation states of +2, +3, +4, +5, and +6. In which state will the element be most likely to form a covalent bond with chlorine?
A) +2
B) +3
C) +4
D) +5
E) +6
A) +2
B) +3
C) +4
D) +5
E) +6

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following transition elements can have an oxidation number of +7?
A) V
B) Cr
C) Mn
D) Fe
E) Co
A) V
B) Cr
C) Mn
D) Fe
E) Co

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
12
How many unpaired electrons are there in the Fe3+ ion?
A) 5
B) 4
C) 3
D) 2
E) 1
A) 5
B) 4
C) 3
D) 2
E) 1

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
13
A feature of transition metal chemistry is that these elements exhibit multiple oxidation states. Which one of the following elements exhibits the smallest number of different oxidation states?
A) Ti
B) Cr
C) Mn
D) Co
E) Zn
A) Ti
B) Cr
C) Mn
D) Co
E) Zn

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following will be paramagnetic?
A) V5+
B) Ni2+
C) Mn7+
D) Ti4+
E) Zn
A) V5+
B) Ni2+
C) Mn7+
D) Ti4+
E) Zn

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following transition elements can achieve the largest oxidation number?
A) chromium, Cr, Group 6B(6)
B) manganese, Mn, Group 7B(7)
C) iron, Fe, Group 8B(8)
D) cobalt, Co, Group 8B(9)
E) zinc, Zn, Group 2B(12)
A) chromium, Cr, Group 6B(6)
B) manganese, Mn, Group 7B(7)
C) iron, Fe, Group 8B(8)
D) cobalt, Co, Group 8B(9)
E) zinc, Zn, Group 2B(12)

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
16
The ground state electronic configuration of Cr2+ is
A) [Ar]4s13d5.
B) [Ar]4s23d4.
C) [Ar]3d4.
D) [Ar]4s13d3.
E) [Ar]4s23d2.
A) [Ar]4s13d5.
B) [Ar]4s23d4.
C) [Ar]3d4.
D) [Ar]4s13d3.
E) [Ar]4s23d2.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
17
The ground state electronic configuration of Zn2+ is
A) [Ar]4s23d8.
B) [Ar]4s23d10.
C) [Ar]4s13d9.
D) [Ar]3d10.
E) [Ar]3d8.
A) [Ar]4s23d8.
B) [Ar]4s23d10.
C) [Ar]4s13d9.
D) [Ar]3d10.
E) [Ar]3d8.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following will be diamagnetic?
A) Ni2+
B) Cr2+
C) Mn2+
D) Co3+
E) Ti4+
A) Ni2+
B) Cr2+
C) Mn2+
D) Co3+
E) Ti4+

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
19
If M represents a transition element, which of the following oxides should be the least basic?
A) MO
B) M2O
C) M2O3
D) MO2
E) MO3
A) MO
B) M2O
C) M2O3
D) MO2
E) MO3

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
20
Which one of the following has the ground state electron configuration [Ar]3d104s1?
A) In+
B) Cd2+
C) Ag+
D) Ag
E) Cu
A) In+
B) Cd2+
C) Ag+
D) Ag
E) Cu

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
21
Chromium and manganese are among the transition elements that form several different oxides. Which of the following statements characterize these oxides?
A) As the oxidation number on the metal increases, the valence-state electronegativity increases and the oxides change from acidic to basic.
B) As the oxidation number on the metal increases, the valence-state electronegativity increases and the oxides change from basic to acidic.
C) As the oxidation number on the metal increases, the valence-state electronegativity decreases and the oxides change from acidic to basic.
D) As the oxidation number on the metal increases, the valence-state electronegativity decreases and the oxides change from basic to acidic.
E) None of the above statements is correct.
A) As the oxidation number on the metal increases, the valence-state electronegativity increases and the oxides change from acidic to basic.
B) As the oxidation number on the metal increases, the valence-state electronegativity increases and the oxides change from basic to acidic.
C) As the oxidation number on the metal increases, the valence-state electronegativity decreases and the oxides change from acidic to basic.
D) As the oxidation number on the metal increases, the valence-state electronegativity decreases and the oxides change from basic to acidic.
E) None of the above statements is correct.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
22
What is the coordination number of cobalt in the complex ion [Co(en)Cl4]-? (en = ethylenediamine)
A) 1
B) 2
C) 4
D) 6
E) 8
A) 1
B) 2
C) 4
D) 6
E) 8

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
23
The compound K3[Fe(CN)6] is used in calico printing and wool dyeing. Give its systematic name.
A) potassium iron(III) hexacyanate
B) tripotassium iron(III) hexacyanate
C) potassium hexacyanoferrate(III)
D) potassium hexacyanideferrate
E) none of the above
A) potassium iron(III) hexacyanate
B) tripotassium iron(III) hexacyanate
C) potassium hexacyanoferrate(III)
D) potassium hexacyanideferrate
E) none of the above

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following should be the strongest reducing agent?
A) Fe
B) Ru
C) Os
D) Re
E) Cu
A) Fe
B) Ru
C) Os
D) Re
E) Cu

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
25
Aluminum reacts with oxygen in the air to form a protective oxide coating. Silver also reacts with compounds in air to form a black coating. What substance is formed?
A) silver oxide
B) silver chloride
C) silver sulfide
D) silver carbonate
E) silver nitride
A) silver oxide
B) silver chloride
C) silver sulfide
D) silver carbonate
E) silver nitride

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
26
Give the systematic name for [Cu(NH3)4]Cl2.
A) dichlorotetraamminecuprate(II)
B) tetraamminecopper(II) chloride
C) copper(II) ammonium chloride
D) tetraaminocopper(II) chloride
E) none of the above
A) dichlorotetraamminecuprate(II)
B) tetraamminecopper(II) chloride
C) copper(II) ammonium chloride
D) tetraaminocopper(II) chloride
E) none of the above

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
27
When the ethylenediaminetetraacetate ion (EDTA4-) forms a complex with a transition metal ion, how many electrons does it normally donate to the metal?
A) 4
B) 6
C) 8
D) 10
E) 12
A) 4
B) 6
C) 8
D) 10
E) 12

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
28
A certain transition metal complex has the formula MX42+. If the metal ion has a d8 electron configuration, what is the shape of the complex?
A) octahedral
B) square pyramid
C) tetrahedral
D) trigonal pyramid
E) square planar
A) octahedral
B) square pyramid
C) tetrahedral
D) trigonal pyramid
E) square planar

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the oxidation states of chromium has the largest valence-state electronegativity?
A) chromium(0)
B) chromium(II)
C) chromium(III)
D) chromium(IV)
E) chromium(VI)
A) chromium(0)
B) chromium(II)
C) chromium(III)
D) chromium(IV)
E) chromium(VI)

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
30
The oxidation and coordination numbers of cobalt in the compound [Co(NH3)5Cl]Cl2 are, respectively:
A) 2 and 6.
B) 2 and 8.
C) 3 and 6.
D) 3 and 8.
E) none of the above
A) 2 and 6.
B) 2 and 8.
C) 3 and 6.
D) 3 and 8.
E) none of the above

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
31
A characteristic of ligands is that
A) they are Lewis acids.
B) they are Lewis bases.
C) they are ions.
D) they are electron pair acceptors.
E) they are Brønsted-Lowry acids.
A) they are Lewis acids.
B) they are Lewis bases.
C) they are ions.
D) they are electron pair acceptors.
E) they are Brønsted-Lowry acids.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
32
In the formation of a transition metal complex, the central metal atom or ion acts as
A) an Arrhenius acid.
B) a Bronsted-Lowry acid.
C) a Bronsted-Lowry base.
D) a Lewis acid.
E) a Lewis base.
A) an Arrhenius acid.
B) a Bronsted-Lowry acid.
C) a Bronsted-Lowry base.
D) a Lewis acid.
E) a Lewis base.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
33
In the compound K[Co(C2O4)2(H2O)2] (where C2O42- = oxalate) the oxidation number and coordination number of cobalt are, respectively:
A) -1 and 4.
B) -1 and 6.
C) 3 and 4.
D) 3 and 6.
E) 1 and 6.
A) -1 and 4.
B) -1 and 6.
C) 3 and 4.
D) 3 and 6.
E) 1 and 6.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
34
Mercury(II) compounds are assimilated into the food chain because
A) they are ionic substances that are concentrated in cell tissue.
B) they complex with compounds in the blood which carries them to the muscle tissue of organisms.
C) they are incorporated into bone structure.
D) they are very soluble in water and easily ingested.
E) they are nonpolar substances that are concentrated in fatty tissues as they move up the food chain.
A) they are ionic substances that are concentrated in cell tissue.
B) they complex with compounds in the blood which carries them to the muscle tissue of organisms.
C) they are incorporated into bone structure.
D) they are very soluble in water and easily ingested.
E) they are nonpolar substances that are concentrated in fatty tissues as they move up the food chain.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following will be the strongest oxidizing agent?
A) Cr
B) Cr(II)
C) Cr(III)
D) Cr(IV)
E) Cr(VI)
A) Cr
B) Cr(II)
C) Cr(III)
D) Cr(IV)
E) Cr(VI)

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
36
In the compound [Ni(en)2(H2O)2]SO4 (where en = ethylenediamine) the oxidation number and coordination number of nickel are, respectively:
A) 2 and 6.
B) 4 and 6.
C) 6 and 6.
D) 2 and 4.
E) 4 and 4.
A) 2 and 6.
B) 4 and 6.
C) 6 and 6.
D) 2 and 4.
E) 4 and 4.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following coordination numbers applies to octahedral complexes?
A) 4
B) 5
C) 6
D) 8
E) none of the above
A) 4
B) 5
C) 6
D) 8
E) none of the above

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is not a property of silver that is important in black and white photography?
A) Silver halides are not soluble in water.
B) Silver metal is easily oxidized.
C) Silver halides undergo a redox reaction when exposed to light.
D) Silver ions will form stable, water-soluble complex ions.
E) Silver atoms catalyze the reduction of silver ions.
A) Silver halides are not soluble in water.
B) Silver metal is easily oxidized.
C) Silver halides undergo a redox reaction when exposed to light.
D) Silver ions will form stable, water-soluble complex ions.
E) Silver atoms catalyze the reduction of silver ions.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
39
Which one of the following normally acts as a bidentate ligand in complexes with transition metal ions?
A) CN-
B) EDTA4-
C) SCN-
D) ethylene diamine
E) ethylene, C2H4
A) CN-
B) EDTA4-
C) SCN-
D) ethylene diamine
E) ethylene, C2H4

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is considered a bidentate ligand?
A) cyanide, CN-
B) thiocyanate, SCN-
C) oxalate, C2O42-
D) nitrite, NO2-
E) hydroxide, OH-
A) cyanide, CN-
B) thiocyanate, SCN-
C) oxalate, C2O42-
D) nitrite, NO2-
E) hydroxide, OH-

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
41
Consider the following structures (1 and 2 are octahedral; 3 and 4 are square planar). 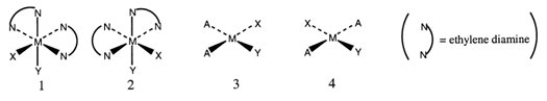 Which one of the following statements about the above structures is correct?
Which one of the following statements about the above structures is correct?
A) 1 and 2 are superimposable.
B) 1 and 2 are geometric isomers.
C) 3 and 4 are structural isomers.
D) 3 and 4 are optical isomers.
E) 3 and 4 are geometric isomers.
 Which one of the following statements about the above structures is correct?
Which one of the following statements about the above structures is correct?A) 1 and 2 are superimposable.
B) 1 and 2 are geometric isomers.
C) 3 and 4 are structural isomers.
D) 3 and 4 are optical isomers.
E) 3 and 4 are geometric isomers.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
42
According to Valence Bond theory, in the square planar Ni(CN)42- complex ion, the orbital hybridization pattern is
A) sp3.
B) dsp2.
C) d2sp.
D) d2sp3.
E) none of the above.
A) sp3.
B) dsp2.
C) d2sp.
D) d2sp3.
E) none of the above.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
43
Write the formula for pentaamminechlorocobalt(III) chloride.
A) [Co(NH3)5Cl]Cl
B) [Co(NH3)5Cl]Cl2
C) [Co(NH3)5Cl]Cl3
D) [Co(NH3)5Cl]Cl4
E) none of the above
A) [Co(NH3)5Cl]Cl
B) [Co(NH3)5Cl]Cl2
C) [Co(NH3)5Cl]Cl3
D) [Co(NH3)5Cl]Cl4
E) none of the above

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
44
In a coordination compound involving a complex ion of square planar geometry, which of the following types of isomerism is/are never possible?
A) geometric
B) optical
C) linkage
D) coordination
E) more than one of the above
A) geometric
B) optical
C) linkage
D) coordination
E) more than one of the above

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
45
Give the systematic name for Cr(CO)3(NH3)3.
A) chromiumtriaminotricarbonyl
B) triamminechromium carbonate
C) triamminetricarbonylchromate(0)
D) triamminetricarbonylchromium(0)
E) none of the above
A) chromiumtriaminotricarbonyl
B) triamminechromium carbonate
C) triamminetricarbonylchromate(0)
D) triamminetricarbonylchromium(0)
E) none of the above

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
46
The crystal field splitting energy, ,
A) is larger for tetrahedral complexes than for octahedral complexes.
B) depends on the metal but not on the ligand.
C) determines the color of a complex.
D) is larger for ionic ligands like chloride than for molecular ligands like carbon monoxide, CO.
E) determines the charge of a complex.
A) is larger for tetrahedral complexes than for octahedral complexes.
B) depends on the metal but not on the ligand.
C) determines the color of a complex.
D) is larger for ionic ligands like chloride than for molecular ligands like carbon monoxide, CO.
E) determines the charge of a complex.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
47
According to valence bond theory, what would be the set of hybrid orbitals used when a Period 4 transition metal forms a tetrahedral complex?
A) d2sp
B) dsp2
C) dsp3
D) sp3
E) d2p2
A) d2sp
B) dsp2
C) dsp3
D) sp3
E) d2p2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following species could exist as isomers?
A) [Co(H2O)4Cl2]+
B) [Pt(NH3)Br3]-
C) [Pt(en)Cl2]
D) [Pt(NH3)3Cl]+
E) none of the above
A) [Co(H2O)4Cl2]+
B) [Pt(NH3)Br3]-
C) [Pt(en)Cl2]
D) [Pt(NH3)3Cl]+
E) none of the above

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following ions could exist in only the high-spin state in an octahedral complex?
A) Cr2+
B) Mn4+
C) Fe3+
D) Co3+
E) Ni2+
A) Cr2+
B) Mn4+
C) Fe3+
D) Co3+
E) Ni2+

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following ions could exist in either the high-spin or low-spin state in an octahedral complex?
A) Sc3+
B) Ni2+
C) Mn2+
D) Ti4+
E) Zn2+
A) Sc3+
B) Ni2+
C) Mn2+
D) Ti4+
E) Zn2+

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
51
Consider the following octahedral complex structures, each involving ethylene diamine and two different, unidentate ligands X and Y. 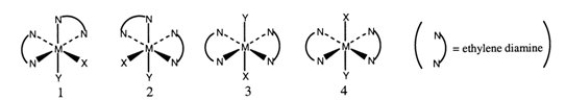 Which one, if any, of the following is a pair of optical isomers?
Which one, if any, of the following is a pair of optical isomers?
A) 1 and 2
B) 1 and 3
C) 1 and 4
D) 3 and 4
E) none of the above
 Which one, if any, of the following is a pair of optical isomers?
Which one, if any, of the following is a pair of optical isomers?A) 1 and 2
B) 1 and 3
C) 1 and 4
D) 3 and 4
E) none of the above

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
52
Write the formula for sodium tetracyanonickelate(II).
A) Na[Ni(CN)4]
B) Na[Ni(CN)4]2
C) Na2[Ni(CN)4]
D) Na4[Ni(CN)4]
E) none of the above
A) Na[Ni(CN)4]
B) Na[Ni(CN)4]2
C) Na2[Ni(CN)4]
D) Na4[Ni(CN)4]
E) none of the above

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
53
Consider the following octahedral complex structures, each involving ethylene diamine and two different, unidentate ligands X and Y. 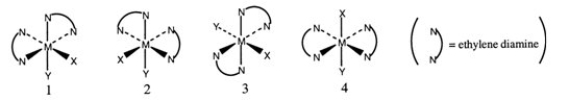 Which one of the following statements about these structures is incorrect?
Which one of the following statements about these structures is incorrect?
A) Structures 1 and 2 are optical isomers.
B) Structures 1 and 3 are optical isomers.
C) Structures 1 and 3 are different complexes.
D) Structures 1 and 4 are geometrical isomers.
E) Structures 3 and 4 are the same complex.
 Which one of the following statements about these structures is incorrect?
Which one of the following statements about these structures is incorrect?A) Structures 1 and 2 are optical isomers.
B) Structures 1 and 3 are optical isomers.
C) Structures 1 and 3 are different complexes.
D) Structures 1 and 4 are geometrical isomers.
E) Structures 3 and 4 are the same complex.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
54
Write the formula for diamminedichloroethylenediaminecobalt(III) bromide.
A) [CoCl2(en)(NH3)2]Br
B) [CoCl2(en)(NH3) 2]Br2
C) [CoCl2(en)2(NH3)2]Br
D) [CoCl2(en)2(NH3) 2]Br2
E) none of the above
A) [CoCl2(en)(NH3)2]Br
B) [CoCl2(en)(NH3) 2]Br2
C) [CoCl2(en)2(NH3)2]Br
D) [CoCl2(en)2(NH3) 2]Br2
E) none of the above

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
55
According to valence bond theory, what would be the set of hybrid orbitals used when a Period 4 transition metal forms a square planar complex?
A) d2sp
B) d2p2
C) dsp3
D) sp3
E) dsp2
A) d2sp
B) d2p2
C) dsp3
D) sp3
E) dsp2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
56
In the presence of a strong octahedral ligand field, the number of unpaired electrons in Co(III) will be
A) 0.
B) 2.
C) 4.
D) 6.
E) none of the above.
A) 0.
B) 2.
C) 4.
D) 6.
E) none of the above.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following octahedral complexes should have the largest crystal field splitting energy, ?
A) [Cr(H2O)6]3+
B) [Cr(SCN)6]3-
C) [Cr(NH3)6]3+
D) [Cr(CN)6]3-
E) [Cr(en)3]3+ (en = ethylenediamine)
A) [Cr(H2O)6]3+
B) [Cr(SCN)6]3-
C) [Cr(NH3)6]3+
D) [Cr(CN)6]3-
E) [Cr(en)3]3+ (en = ethylenediamine)

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
58
Give the systematic name for [CoCl3(H2O)]-.
A) cobalt(II) chloride monohydrate
B) aquatrichlorocobalt(II)
C) aquatrichlorocobaltate(II)
D) aquatrichlorocobaltite(I)
E) none of the above
A) cobalt(II) chloride monohydrate
B) aquatrichlorocobalt(II)
C) aquatrichlorocobaltate(II)
D) aquatrichlorocobaltite(I)
E) none of the above

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
59
In the spectrochemical series, which one of the following ligands has the strongest field?
A) H2O
B) CN-
C) NH3
D) OH-
E) Cl-
A) H2O
B) CN-
C) NH3
D) OH-
E) Cl-

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following ligands could participate in linkage isomerism?
A) NH3
B) H2O
C) NH4+
D) NO2-
E) ethylenediamine
A) NH3
B) H2O
C) NH4+
D) NO2-
E) ethylenediamine

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
61
The M2+ ions of the first transition series of elements all have the general electronic configuration [Ar]4s23dx, where x is an integer from 1 to 8.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
62
Give one important use of the following metals or their compounds.
a. chromium
b. manganese
c. mercury
d. silver
a. chromium
b. manganese
c. mercury
d. silver

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following ligands is most likely to form a high spin octahedral complex with cobalt(II)?
A) CN-
B) en (ethylenediamine)
C) NO2-
D) CO
E) I-
A) CN-
B) en (ethylenediamine)
C) NO2-
D) CO
E) I-

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
64
What geometry is particularly common for complexes of d10 metal ions?

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
65
a. How can the formation of a complex ion be described in terms of a theory of acids and bases?
b. What is the essential requirement for a molecule or ion to act as a ligand?
b. What is the essential requirement for a molecule or ion to act as a ligand?

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following ligands is most likely to form a low-spin octahedral complex with iron(III)?
A) Cl-
B) H2O
C) NH3
D) OH-
E) CO
A) Cl-
B) H2O
C) NH3
D) OH-
E) CO

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
67
All atoms of the first transition series of elements have the ground state electronic configuration [Ar]4s23dx, where x is an integer from 1 to 10.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
68
Apply the valence bond theory to predict the electronic structure and hybridization pattern of chromium in the complex ion Cr(NH3)63+.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
69
The Cu2+ ion has 1 unpaired electron.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
70
The dxy and the dx2-y2 orbitals both lie in the xy plane, yet for a metal ion in an octahedral complex the energy of the dxy orbital is lower than that of the dx2-y2orbital. Explain this using the arguments of crystal field theory.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
71
The ground state electron configuration of a transition element atom cannot have more than one incomplete subshell.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
72
How many unpaired electrons will there be in a high-spin octahedral complex of Fe(II)?
A) 0
B) 2
C) 4
D) 6
E) none of the above
A) 0
B) 2
C) 4
D) 6
E) none of the above

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
73
What is the difference between a coordination compound and a complex ion?

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
74
Iron(III) forms an octahedral complex with the ligand CN-. How many unpaired electrons are in the d orbitals of iron?
A) 1
B) 3
C) 5
D) 7
E) none of the above
A) 1
B) 3
C) 5
D) 7
E) none of the above

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
75
a. Explain how the crystal field theory can use the magnitude of the splitting energy to provide an explanation of the color and magnetic properties of octahedral complexes.
b. In promoting an electron from the t2g set of orbitals to the eg set, an octahedral complex absorbs a photon with a wavelength of 523 nm. Calculate the value of in the complex, in kJ/mol.
b. In promoting an electron from the t2g set of orbitals to the eg set, an octahedral complex absorbs a photon with a wavelength of 523 nm. Calculate the value of in the complex, in kJ/mol.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
76
The compound Rh(CO)(H)(PH3)2 forms cis and trans isomers. Use this information to predict the geometry of this complex, and draw the geometric isomers.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
77
If a solution absorbs green light, what is its likely color?
A) red
B) violet
C) orange
D) yellow
E) blue
A) red
B) violet
C) orange
D) yellow
E) blue

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
78
Why is the +2 oxidation state so common among transition elements?

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
79
a. State the requirement for two molecules to be optical isomers.
b. A complex ion MABCD2+ (where A, B, C, and D are different unidentate ligands) rotates the plane of polarized light. Deduce the geometry of the complex and draw the optical isomers of this ionic formula.
b. A complex ion MABCD2+ (where A, B, C, and D are different unidentate ligands) rotates the plane of polarized light. Deduce the geometry of the complex and draw the optical isomers of this ionic formula.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
80
a. How many unpaired 3d electrons will there be in (i) high and (ii) low-spin complexes of Co(II)?
b. How can high and low-spin complexes be recognized and distinguished experimentally?
b. How can high and low-spin complexes be recognized and distinguished experimentally?

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck


