Deck 30: Nuclear Physics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/48
Play
Full screen (f)
Deck 30: Nuclear Physics
1
An archaeologist finds the 14C in a sample of 3.10 g of material to be decaying at 107 counts per second.A modern 1.00-g sample of the same material decays at 151 counts per second.The half-life of 14C is 5730 years.How old is the sample?
A)12,200 years
B)8460 years
C)25,100 years
D)12,600 years
A)12,200 years
B)8460 years
C)25,100 years
D)12,600 years
12,200 years
2
The following masses are known: 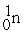 1.008665 u
1.008665 u 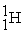 1.007825 u
1.007825 u 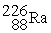 226.025403 u The binding energy of
226.025403 u The binding energy of 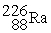 ,in MeV,is closest to:
,in MeV,is closest to:
A)1700
B)1900
C)2100
D)2300
E)2500
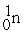 1.008665 u
1.008665 u 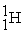 1.007825 u
1.007825 u 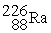 226.025403 u The binding energy of
226.025403 u The binding energy of 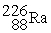 ,in MeV,is closest to:
,in MeV,is closest to:A)1700
B)1900
C)2100
D)2300
E)2500
1700
3
A summary of the nuclear reactions that power our sun can be written as 4p 4He + 2e-,with masses of 938.272 MeV/c2 for proton,3727.38 MeV/c2 for helium,and 0.511 MeV/c2 for electrons.If this is the reaction that occurs,how much energy is released?
A)24.69 MeV
B)28.3 MeV
C)2790.13 MeV
D)279.01 MeV
A)24.69 MeV
B)28.3 MeV
C)2790.13 MeV
D)279.01 MeV
24.69 MeV
4
The first nuclear reaction observed saw an alpha particle (Z = 2)interact with a nitrogen nucleus in air (Z = 7)to produce a proton.The energy of the alpha particle was 55 MeV,enough to enable the nuclei to touch in spite of the Coulomb repulsion.What distance between the centers of the alpha particle and the nitrogen was reached? This determines a nuclear radius for nitrogen.Use 1 fm = 10-15 m.
A)0.37 fm
B)0.18 fm
C)0.05 fm
D)158.40 fm
A)0.37 fm
B)0.18 fm
C)0.05 fm
D)158.40 fm

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
5
An air sample is contaminated with 15O,which has a half-life of 2.03 minutes.You can pass it through a long pipe to allow it to decay until it can be safely released,at an air speed of 1.1 m/s.How long must your pipeline be for the sample to decay to 3% of its original activity?
A)678 m
B)8 m
C)7 m
D)2 m
A)678 m
B)8 m
C)7 m
D)2 m

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
6
About how many days are required for a radioactive sample,with an initial activity of 105 Bq,to decay to an activity of 100 Bq? The half-life of the material is 4.5 days.
A)about 45 days
B)about 36 days
C)about 54 days
D)about 31 days
A)about 45 days
B)about 36 days
C)about 54 days
D)about 31 days

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
7
Today,uranium contains 0.72% 235U (half-life = 0.70 billion years)and 99.28% 238U (half-life = 4.5 billion years).At a time 1.9 billion years ago,what was the fraction of 235U in uranium?
A)3.53%
B)4.72%
C)4.90%
D)6.75%
A)3.53%
B)4.72%
C)4.90%
D)6.75%

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
8
The maximum permissible workday dose for occupational exposure to radiation is 11 mrem.A 77 kg laboratory technician absorbs 2.3 mJ of 0.5 MeV gamma rays in a work day.The relative biological efficiency (RBE)for gamma rays is 1.00.The number of gamma-ray photons absorbed by the technician in a workday is closest to:
A)3 × 1010
B)3 × 109
C)3 × 108
D)1 × 109
E)1 × 108
A)3 × 1010
B)3 × 109
C)3 × 108
D)1 × 109
E)1 × 108

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
9
How much energy is released in the total fission of 2.0 g of 235U? The average energy per fission is 200.0 MeV.
A)1.6 × 1011 J
B)3.9 × 1013 J
C)1.6 × 105 J
D)3.9 × 1010 J
A)1.6 × 1011 J
B)3.9 × 1013 J
C)1.6 × 105 J
D)3.9 × 1010 J

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
10
The maximum permissible workday dose for occupational exposure to radiation is 18 mrem.A 54 kg laboratory technician absorbs 2.6 mJ of 0.3 MeV gamma rays in a work day.The relative biological efficiency (RBE)for gamma rays is 1.00.The ratio of the equivalent dosage received by the technician to the maximum permissible equivalent dosage is closest to:
A)0.27
B)0.29
C)0.32
D)0.35
E)0.37
A)0.27
B)0.29
C)0.32
D)0.35
E)0.37

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
11
In a head-on collision,an alpha particle (Z = 2)of energy 8.20 MeV bounces straight back from a nucleus of charge 80.0 e.How close were the centers of the objects at closest approach?
A)2.81 × 10-14 m
B)3.39 × 10-12 m
C)6.56 × 10-15 m
D)2.17 × 10-14 m
A)2.81 × 10-14 m
B)3.39 × 10-12 m
C)6.56 × 10-15 m
D)2.17 × 10-14 m

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
12
How much energy is released when 0.600 µg of 3H have decayed to 3He? Use 1 u = 931.494 MeV/c2,M(3He)= 3.01493 u,and M(3H)= 3.01550 u.
A)1.02 × 104 J
B)5.12 × 103 J
C)3.41 × 103 J
D)3.07 × 104 J
A)1.02 × 104 J
B)5.12 × 103 J
C)3.41 × 103 J
D)3.07 × 104 J

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
13
What mass of 14C (half-life = 5730 years)do you need to provide a decay rate of 280.0 Bq?
A)1.70 × 10-12 kg
B)5.38 × 10-19 kg
C)3.84 × 10-20 kg
D)8.68 × 10-13 kg
A)1.70 × 10-12 kg
B)5.38 × 10-19 kg
C)3.84 × 10-20 kg
D)8.68 × 10-13 kg

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
14
An ancient rock is found to contain 40Ar gas,indicating that 77% of the 40K in the rock has decayed since the rock solidified.Any argon would have boiled out of liquid rock.How long ago did the rock solidify? The half-life of 40K is 1.25 billion years.
A)2.6 billion years
B)0.5 billion years
C)1.8 billion years
D)0.3 billion years
A)2.6 billion years
B)0.5 billion years
C)1.8 billion years
D)0.3 billion years

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
15
The material used in nuclear bombs is 239Pu,with a half-life of about 20,000 years.How long must we wait for a buried stockpile of this substance to decay to 4% of its original 239Pu mass?
A)93 thousand years
B)64 thousand years
C)45 thousand years
D)0.80000001 thousand years
A)93 thousand years
B)64 thousand years
C)45 thousand years
D)0.80000001 thousand years

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
16
A hospital patient has been given 131I (half-life = 8.04 days),which decays at 4.2 times the acceptable level for exposure to the general public.How long must the patient wait to reach the acceptable level? Assume that the material merely decays and is not excreted.
A)17 days
B)12 days
C)8.0 days
D)7.2 days
A)17 days
B)12 days
C)8.0 days
D)7.2 days

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
17
Approximately what fraction of the volume of an atom is made up of the nucleus?
A)10-15
B)10-6
C)10-1
D)10-24
A)10-15
B)10-6
C)10-1
D)10-24

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
18
The carbon in your body was formed in nuclear reactions in long-dead stars.How much energy was released when the right number of 4He nuclei combined to make 12C? M(4He)c2 = 3728.40 MeV.
A)7.320 MeV
B)3716 MeV
C)8.424 MeV
D)2.106 MeV
A)7.320 MeV
B)3716 MeV
C)8.424 MeV
D)2.106 MeV

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
19
A nuclear bomb explosion results in a mass decrease of about 2.10 g between the initial and the final ingredients.How much energy is released?
A)1.89 × 1014 J
B)6.30 × 105 J
C)1.89 × 1013 J
D)2.25 × 1012 J
A)1.89 × 1014 J
B)6.30 × 105 J
C)1.89 × 1013 J
D)2.25 × 1012 J

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
20
The decay constant of a radioactive nuclide is 1.6 ×10-3s-1.At a given instant,the number of atoms of the radioactive nuclide is 1.85 ×1012.The number of atoms of the nuclide that remain after a time interval of 30 minutes is closest to:
A)1.04 × 1011
B)1.14 × 1011
C)1.26 × 1011
D)1.38 × 1011
E)1.52 × 1011
A)1.04 × 1011
B)1.14 × 1011
C)1.26 × 1011
D)1.38 × 1011
E)1.52 × 1011

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
21
If a 20 kg brick is made entirely of radioactive atoms of half-life 5 days,at the end of 5 days this brick will have a mass of 10 kg.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
22
A positron (or antielectron)is made to stop in a sample of matter.Soon after,how many gamma rays are observed with what energy each?
A)two,0.511 MeV each
B)one,0.511 MeV
C)none
D)two,13.6 eV each
E)one,13.6 eV
A)two,0.511 MeV each
B)one,0.511 MeV
C)none
D)two,13.6 eV each
E)one,13.6 eV

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
23
Modern nuclear bomb tests have created an extra high level of 14C in our atmosphere.When future archaeologists date samples from this era,without knowing of this testing,will their dates be too young? Too old? Correct? If correct,why?
A)too young
B)too old
C)Correct,since 14C from bomb tests is different from that produced naturally.
D)Correct,because modern biological materials do not gather 14C from bomb tests.
A)too young
B)too old
C)Correct,since 14C from bomb tests is different from that produced naturally.
D)Correct,because modern biological materials do not gather 14C from bomb tests.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
24
The primary reason very large nuclei are unstable is due to
A)the cumulative repulsive force amongst the protons.
B)the cumulative attractive force between the protons and the orbiting electrons.
C)the repulsive force between the neutrons and the protons.
A)the cumulative repulsive force amongst the protons.
B)the cumulative attractive force between the protons and the orbiting electrons.
C)the repulsive force between the neutrons and the protons.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
25
A radioactive nuclide of atomic number Z emits an electron,then the daughter nuclide emits a gamma ray.What is the atomic number of the resulting nuclide after both processes?
A)Z+1
B)Z-1
C)Z-2
D)Z-3
A)Z+1
B)Z-1
C)Z-2
D)Z-3

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
26
Why do heavy nuclei contain more neutrons than protons?
A)Neutrons dilute the electric repulsion of the protons.
B)Neutrons are lighter than protons.
C)Neutrons are heavier than protons
D)Neutrons are radioactive,and so are heavy nuclei.
A)Neutrons dilute the electric repulsion of the protons.
B)Neutrons are lighter than protons.
C)Neutrons are heavier than protons
D)Neutrons are radioactive,and so are heavy nuclei.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
27
If an element emits one alpha particle,its atomic number decreases by
A)two.
B)one.
C)four.
D)zero.
A)two.
B)one.
C)four.
D)zero.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
28
The majority of known nuclei are stable.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
29
Neutrons are slightly more massive than protons.If the mass of a neutron were smaller than its actual value,yet still more massive than a proton,a nucleus with a very high atomic number would most likely contain
A)more neutrons than the actual number.
B)fewer neutrons than the actual number.
C)the same number of neutrons as the actual number.
A)more neutrons than the actual number.
B)fewer neutrons than the actual number.
C)the same number of neutrons as the actual number.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
30
Stable nuclei have equal numbers of protons and neutrons.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
31
The binding energy per nucleon increases steadily with the mass number of the nucleus.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
32
In beta-plus decay,a proton in the nucleus is converted to a neutron,a positron,and a neutrino.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
33
Two radioactive nuclides X and Y both decay to stable products.The half-life of X is about a day,while that of Y is about a week.Suppose a radioactive sample consists of a mixture of these two nuclides.If the mixture is such that the activities arising from X and Y are initially equal,then a few days later the activity of the sample will be due
A)predominantly to nuclide Y.
B)predominantly to nuclide X.
C)entirely to nuclide Y.
D)to nuclides X and Y equally.
A)predominantly to nuclide Y.
B)predominantly to nuclide X.
C)entirely to nuclide Y.
D)to nuclides X and Y equally.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
34
Neodymium 144Nd is a nuclide that undergoes alpha decay.The nuclide that is the product of the decay is:
A)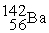
B)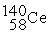
C)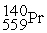
D)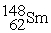
E)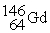
A)
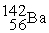
B)
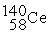
C)
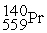
D)
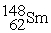
E)
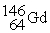

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
35
In beta-minus decay,the number of protons in a nucleus increases but the number of nucleons does not change.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
36
Scandium 44Sc decays by emitting a positron.The nuclide that is the product of the decay is:
A)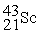
B)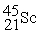
C)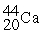
D)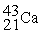
E)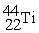
A)
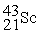
B)
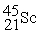
C)
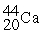
D)
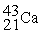
E)
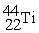

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
37
Because neutrons and protons are approximately 1837 times as massive as electrons,the strong force acts on them approximately 1837 times as much as it acts on electrons.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
38
In massive stars,three helium atoms fuse together,forming a carbon nucleus.This reaction heats the core of the star.The net mass of the three helium nuclei must therefore be
A)higher than that of the carbon nucleus.
B)less than that of the carbon nucleus.
C)the same as that of the carbon nucleus (mass is always conserved).
A)higher than that of the carbon nucleus.
B)less than that of the carbon nucleus.
C)the same as that of the carbon nucleus (mass is always conserved).

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
39
Nuclei that are all isotopes of an element all have the same
A)number of protons.
B)mass.
C)number of nucleons.
D)number of neutrons.
A)number of protons.
B)mass.
C)number of nucleons.
D)number of neutrons.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
40
If half of a radioactive substance decays in the first 10 s,the other half will decay in the next 10 s.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following is not true of the nuclear force?
A)The nuclear force has a short range,of the order of nuclear dimensions.
B)For two protons in close proximity,the nuclear force and the electric force have comparable magnitudes.
C)The nuclear force does not depend on charge.
D)A nucleon in a large nucleus interacts via the nuclear force only with nearby nucleons,not with ones far away in the nucleus.
E)The nuclear force favors binding of pairs of protons or neutrons with opposite spin angular momenta.
A)The nuclear force has a short range,of the order of nuclear dimensions.
B)For two protons in close proximity,the nuclear force and the electric force have comparable magnitudes.
C)The nuclear force does not depend on charge.
D)A nucleon in a large nucleus interacts via the nuclear force only with nearby nucleons,not with ones far away in the nucleus.
E)The nuclear force favors binding of pairs of protons or neutrons with opposite spin angular momenta.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
42
The stability of 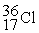 with respect to alpha,beta-plus,and beta-minus decay is to be determined.The following atomic masses in amu are known:
with respect to alpha,beta-plus,and beta-minus decay is to be determined.The following atomic masses in amu are known: 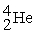 4.002603
4.002603 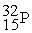 31.973907
31.973907 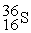 35.967081
35.967081 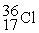 35.968307
35.968307 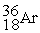 35.967546 The
35.967546 The 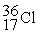 nuclide is
nuclide is
A)not subject to alpha,beta-plus,or beta-minus decay.
B)subject to alpha decay only.
C)subject to beta-plus decay only.
D)subject to beta-minus decay only.
E)subject to beta-plus or beta-minus decay,but not to alpha decay.
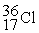 with respect to alpha,beta-plus,and beta-minus decay is to be determined.The following atomic masses in amu are known:
with respect to alpha,beta-plus,and beta-minus decay is to be determined.The following atomic masses in amu are known: 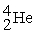 4.002603
4.002603 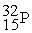 31.973907
31.973907 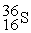 35.967081
35.967081 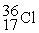 35.968307
35.968307 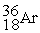 35.967546 The
35.967546 The 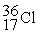 nuclide is
nuclide isA)not subject to alpha,beta-plus,or beta-minus decay.
B)subject to alpha decay only.
C)subject to beta-plus decay only.
D)subject to beta-minus decay only.
E)subject to beta-plus or beta-minus decay,but not to alpha decay.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
43
The half-lives of cobalt-60 and strontium-90 are 5.3 years and 28 years,respectively.Suppose that samples of cobalt-60 and strontium-90 are such that they initially have the same activity.What will be true of the numbers of cobalt-60 and strontium-90 nuclei in these samples?
A)There will be more strontium-90 than cobalt-60 nuclei.
B)There will be equal numbers of cobalt-60 and strontium-90 nuclei.
C)There will be more cobalt-60 than strontium-90 nuclei.
D)It is not possible to compare numbers of nuclei without knowing the masses of the samples.
A)There will be more strontium-90 than cobalt-60 nuclei.
B)There will be equal numbers of cobalt-60 and strontium-90 nuclei.
C)There will be more cobalt-60 than strontium-90 nuclei.
D)It is not possible to compare numbers of nuclei without knowing the masses of the samples.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
44
Suppose the half-life of some element is 2 days.If you purchase 10 grams of the element (which was produced in a laboratory 4 days ago),how much of this element would you have 3 days after you purchased it?
A)more than 2.5 grams but less than 5 grams
B)2.5 grams
C)more than 1.25 grams but less than 2.5 grams
D)1.25 grams
E)less than 1.25 grams
A)more than 2.5 grams but less than 5 grams
B)2.5 grams
C)more than 1.25 grams but less than 2.5 grams
D)1.25 grams
E)less than 1.25 grams

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
45
The stability of 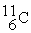 with respect to alpha,beta-plus,and beta-minus decay is to be determined.The following atomic masses in amu are known:
with respect to alpha,beta-plus,and beta-minus decay is to be determined.The following atomic masses in amu are known: 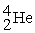 4.002603
4.002603 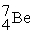 7.016928
7.016928 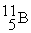 11.009305
11.009305 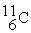 11.011433
11.011433 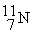 11.026742 The
11.026742 The 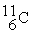 nuclide is
nuclide is
A)not subject to alpha,beta-plus,or beta-minus decay.
B)subject to alpha decay only.
C)subject to beta-plus decay only.
D)subject to beta-minus decay only.
E)subject to beta-plus or beta-minus decay,but not to alpha decay.
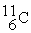 with respect to alpha,beta-plus,and beta-minus decay is to be determined.The following atomic masses in amu are known:
with respect to alpha,beta-plus,and beta-minus decay is to be determined.The following atomic masses in amu are known: 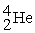 4.002603
4.002603 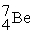 7.016928
7.016928 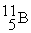 11.009305
11.009305 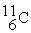 11.011433
11.011433 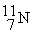 11.026742 The
11.026742 The 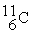 nuclide is
nuclide isA)not subject to alpha,beta-plus,or beta-minus decay.
B)subject to alpha decay only.
C)subject to beta-plus decay only.
D)subject to beta-minus decay only.
E)subject to beta-plus or beta-minus decay,but not to alpha decay.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
46
A radioactive nuclide of atomic number Z emits an alpha particle and the daughter nucleus then emits a beta particle.What is the atomic number of the resulting nuclide?
A)Z-1
B)Z+1
C)Z-2
D)Z-3
A)Z-1
B)Z+1
C)Z-2
D)Z-3

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
47
A stable nucleus contains many protons very close to each other,all positively charged.Why do the protons not fly apart due to mutual Coulomb repulsion?
A)Attractive nuclear forces in the nucleus counteract the effect of the Coulomb forces.
B)There are an equal number of electrons in the nucleus that neutralize the protons.
C)The neutrons in the nucleus shield the protons from each other.
D)The Coulomb force does not operate within nuclei.
A)Attractive nuclear forces in the nucleus counteract the effect of the Coulomb forces.
B)There are an equal number of electrons in the nucleus that neutralize the protons.
C)The neutrons in the nucleus shield the protons from each other.
D)The Coulomb force does not operate within nuclei.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
48
The half-life of cobalt-60 is 5.3 years,while that of strontium-90 is 28 years.Suppose you have a sample of each,such that they initially contain equal numbers of atoms of these nuclides.How will the activities (number of decays per unit time)of the samples compare?
A)The activity of the cobalt-60 sample will be greater.
B)The activities cannot be compared without more information.
C)The activities will be equal.
D)The activity of the strontium-90 sample will be greater.
A)The activity of the cobalt-60 sample will be greater.
B)The activities cannot be compared without more information.
C)The activities will be equal.
D)The activity of the strontium-90 sample will be greater.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck


