Deck 18: The First Law of Thermodynamics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/52
Play
Full screen (f)
Deck 18: The First Law of Thermodynamics
1
How much work is done by 3.00 mol of ideal gas when it triples its volume at a constant temperature of 127°C? The ideal gas constant is R = 8.314 J/mol ∙ K.
A) 12.7 kJ
B) 9.97 kJ
C) 11.0 kJ
D) 15.3 kJ
E) 1.20 kJ
A) 12.7 kJ
B) 9.97 kJ
C) 11.0 kJ
D) 15.3 kJ
E) 1.20 kJ
11.0 kJ
2
The process shown in the pV diagram in the figure is 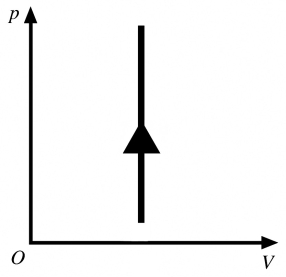
A) adiabatic.
B) isothermal.
C) isochoric.
D) isobaric.

A) adiabatic.
B) isothermal.
C) isochoric.
D) isobaric.
isochoric.
3
An ideal gas is compressed in a well-insulated chamber using a well-insulated piston. This process is
A) isochoric.
B) isothermal.
C) adiabatic.
D) isobaric.
A) isochoric.
B) isothermal.
C) adiabatic.
D) isobaric.
adiabatic.
4
The process shown in the T-V diagram in the figure is an 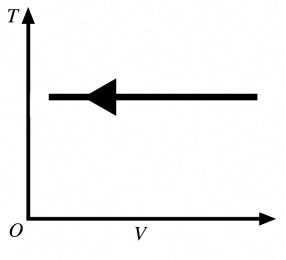
A) adiabatic compression.
B) isothermal compression.
C) isochoric compression.
D) isobaric compression.
E) isothermal expansion.

A) adiabatic compression.
B) isothermal compression.
C) isochoric compression.
D) isobaric compression.
E) isothermal expansion.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
5
The figure shows a pV diagram for 4.3 g of oxygen gas (O2) in a sealed container. The temperature T1 of the gas in state 1 is 21°C. What are the temperatures T3 and T4 of the gas in states 3 and 4? The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K, and the ATOMIC weight of oxygen is 16 g/mol. 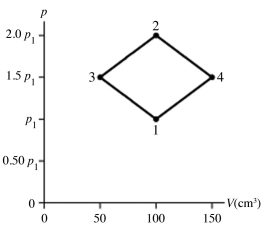
A) -52°C, 390°C
B) 16°C, 47°C
C) 220°C, 660°C
D) 11°C, 32°C

A) -52°C, 390°C
B) 16°C, 47°C
C) 220°C, 660°C
D) 11°C, 32°C

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
6
An ideal gas in a balloon is kept in thermal equilibrium with its constant-temperature surroundings. How much work is done by the gas if the outside pressure is slowly reduced, allowing the balloon to expand to 6.0 times its original size? The balloon initially has a pressure of 645.0 Pa and a volume of 0.10 m3. The ideal gas constant is R = 8.314 J/mol ∙ K.
A) 120 J
B) 390 J
C) -330 J
D) 6.0 J
A) 120 J
B) 390 J
C) -330 J
D) 6.0 J

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
7
The figure shows a pV diagram for 8.3 g of nitrogen gas (N2) in a sealed container. The temperature T1 of the gas in state 1 is 79°C. What are (a) the pressure p1 of the gas in state 1 and (b) the temperature T2 of the gas in state 2? The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K, and the ATOMIC weight of nitrogen is 14 g/mol. 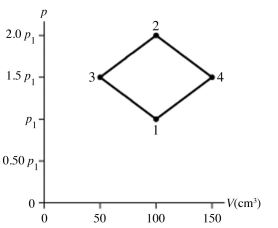
A) (a) 86 atm, (b) 700°C.
B) (a) 19 atm, (b) 700°C.
C) (a) 86 atm, (b) 160°C.
D) (a) 19 atm, (b) 160°C.

A) (a) 86 atm, (b) 700°C.
B) (a) 19 atm, (b) 700°C.
C) (a) 86 atm, (b) 160°C.
D) (a) 19 atm, (b) 160°C.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
8
The process shown in the pV diagram in the figure is an 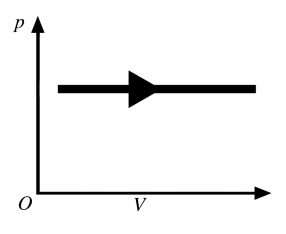
A) adiabatic expansion.
B) isothermal expansion.
C) isochoric expansion.
D) isobaric expansion.
E) isochoric compression.

A) adiabatic expansion.
B) isothermal expansion.
C) isochoric expansion.
D) isobaric expansion.
E) isochoric compression.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
9
A steel container, equipped with a piston, contains 21 mol of an ideal gas at 465 K. The container is compressed isothermally to 90% of its original volume. How much work is done on the gas? The ideal gas constant is R = 8.314 J/mol ∙ K.
A) 8600 J
B) -73,300 J
C) -8500 J
D) 11 J
A) 8600 J
B) -73,300 J
C) -8500 J
D) 11 J

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
10
The figure shows a pV diagram for 0.0066 mol of gas that undergoes the process 1 → 2 → 3. What is the pressure p2. The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K. 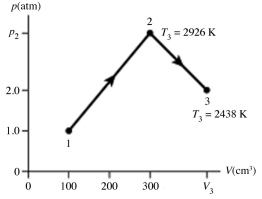
A) 5.3 atm
B) 5.3 × 105 atm
C) 16 atm
D) 1.6 × 106 atm

A) 5.3 atm
B) 5.3 × 105 atm
C) 16 atm
D) 1.6 × 106 atm

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
11
When a fixed amount of ideal gas goes through an isochoric process
A) its internal (thermal) energy does not change.
B) the gas does no work.
C) no heat enters or leaves the gas.
D) its temperature must increase.
E) its pressure must increase.
A) its internal (thermal) energy does not change.
B) the gas does no work.
C) no heat enters or leaves the gas.
D) its temperature must increase.
E) its pressure must increase.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
12
When a fixed amount of ideal gas goes through an isobaric expansion
A) its internal (thermal) energy does not change.
B) the gas does no work.
C) no heat enters or leaves the gas.
D) its temperature must increase.
E) its pressure must increase.
A) its internal (thermal) energy does not change.
B) the gas does no work.
C) no heat enters or leaves the gas.
D) its temperature must increase.
E) its pressure must increase.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
13
When an ideal gas increases in volume at constant pressure, the average kinetic energy of the gas molecules
A) increases.
B) decreases.
C) does not change.
D) may either increase or decrease, depending on whether or not the process is carried out adiabatically.
E) may or may not change, but insufficient information is given to make such a determination.
A) increases.
B) decreases.
C) does not change.
D) may either increase or decrease, depending on whether or not the process is carried out adiabatically.
E) may or may not change, but insufficient information is given to make such a determination.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
14
A container of ideal gas has a movable frictionless piston. This container is placed in a very large water bath and slowly compressed so that the temperature of the gas remains constant and equal to the temperature of the water. Which of the following statements about this gas are true for this process? (There may be more than one correct choice.)
A) Heat leaves the gas during the compression.
B) Since the gas and water are at the same temperature, no heat can flow between them, which makes this an adiabatic compression.
C) The internal (thermal) energy of the gas does not change during the compression.
D) The internal energy of the gas increases during the compression because work is done on the gas.
E) Since the temperature of the gas remains constant, the pressure of the gas must also remain constant.
A) Heat leaves the gas during the compression.
B) Since the gas and water are at the same temperature, no heat can flow between them, which makes this an adiabatic compression.
C) The internal (thermal) energy of the gas does not change during the compression.
D) The internal energy of the gas increases during the compression because work is done on the gas.
E) Since the temperature of the gas remains constant, the pressure of the gas must also remain constant.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
15
The figure (not to scale) shows a pV diagram for 1.8 g of helium gas (He) that undergoes the process 1 → 2 → 3. Find the value of V3. The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K, and the atomic weight of helium is 4.0 g/mol. 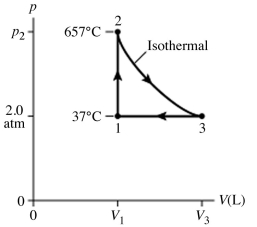
A) 17 L
B) 69 L
C) 34 L
D) 8.6 L

A) 17 L
B) 69 L
C) 34 L
D) 8.6 L

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
16
When a fixed amount of ideal gas goes through an adiabatic expansion
A) its internal (thermal) energy does not change.
B) the gas does no work.
C) no heat enters or leaves the gas.
D) its temperature cannot change.
E) its pressure must increase.
A) its internal (thermal) energy does not change.
B) the gas does no work.
C) no heat enters or leaves the gas.
D) its temperature cannot change.
E) its pressure must increase.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
17
When a gas undergoes an isothermal process, there is
A) no change in the pressure of the gas.
B) no change in the temperature of the gas.
C) no change in the volume of the gas.
D) no work done by (or on) the gas.
E) no heat added to the gas.
A) no change in the pressure of the gas.
B) no change in the temperature of the gas.
C) no change in the volume of the gas.
D) no work done by (or on) the gas.
E) no heat added to the gas.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
18
When a fixed amount of ideal gas goes through an isothermal expansion
A) its internal (thermal) energy does not change.
B) the gas does no work.
C) no heat enters or leaves the gas.
D) its temperature must decrease.
E) its pressure must increase.
A) its internal (thermal) energy does not change.
B) the gas does no work.
C) no heat enters or leaves the gas.
D) its temperature must decrease.
E) its pressure must increase.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
19
An ideal gas increases in temperature from 22°C to 42°C by two different processes. In one process, the temperature increases at constant volume, and in the other process the temperature increases at constant pressure. Which of the following statements about this gas are correct? (There may be more than one correct choice.)
A) The heat required to cause this temperature change is the same for both the constant-volume and the constant-pressure processes.
B) More heat is required for the constant-pressure process than for the constant-volume process.
C) The change in the internal (thermal) energy of the gas is the same for both the constant-volume and the constant-pressure processes.
D) The root-mean-square (thermal) speed of the gas molecules increases more during the constant-volume process than during the constant-pressure process.
A) The heat required to cause this temperature change is the same for both the constant-volume and the constant-pressure processes.
B) More heat is required for the constant-pressure process than for the constant-volume process.
C) The change in the internal (thermal) energy of the gas is the same for both the constant-volume and the constant-pressure processes.
D) The root-mean-square (thermal) speed of the gas molecules increases more during the constant-volume process than during the constant-pressure process.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
20
The figure shows a pV diagram for 0.95 mol of gas that undergoes the process 1 → 2. The gas then undergoes an isochoric heating from point 2 until the pressure is restored to the value it had at point 1. What is the final temperature of the gas? The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K. 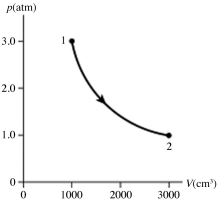
A) -160°C
B) 15°C
C) 390°C
D) 120°C

A) -160°C
B) 15°C
C) 390°C
D) 120°C

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
21
In an isochoric process, the internal (thermal) energy of an ideal gas decreases by 50 J. How much heat is exchanged with the gas during this process?
A) 0.00 J
B) 25 J
C) 50 J
D) -25 J
E) -50 J
A) 0.00 J
B) 25 J
C) 50 J
D) -25 J
E) -50 J

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
22
The temperature of an ideal gas in a sealed 0.40 m3 container is reduced from 400 K to 270 K. The final pressure of the gas is 30 kPA. The molar heat capacity at constant volume of the gas is 28.0 J/mol · K. The work done by the gas is closest to
A) 0.00 kJ.
B) -19 kJ.
C) -25 kJ.
D) 19 kJ.
E) 25 kJ.
A) 0.00 kJ.
B) -19 kJ.
C) -25 kJ.
D) 19 kJ.
E) 25 kJ.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
23
In an isochoric process, the internal (thermal) energy of an ideal gas decreases by 50 J. How much work does the gas do during this process?
A) 0.00 J
B) 25 J
C) 50 J
D) -25 J
E) -50 J
A) 0.00 J
B) 25 J
C) 50 J
D) -25 J
E) -50 J

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
24
During an isothermal process, 5.0 J of heat is removed from an ideal gas. How much work does the gas do during this process?
A) 0.00 J
B) 2.0 J
C) 5.0 J
D) -5.0 J
E) 10 J
A) 0.00 J
B) 2.0 J
C) 5.0 J
D) -5.0 J
E) 10 J

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
25
An expansion process on an ideal diatomic gas has a linear path between the initial and final states on a pV diagram. The initial pressure is 300 kPa, the initial volume is 0.020 m3, and the initial temperature is 390 K. The final pressure is 160 kPa and the final temperature is 310K. The change in the internal (thermal) energy of the gas is closest to
A) -3100 J.
B) -1800 J.
C) 3100 J.
D) 1800 J.
E) 0.00 J.
A) -3100 J.
B) -1800 J.
C) 3100 J.
D) 1800 J.
E) 0.00 J.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
26
3.0 moles of an ideal gas with a molar heat capacity at constant volume of 4.9 cal/(mol∙K) and a molar heat capacity at constant pressure of 6.9 cal/(mol∙K) starts at 300 K and is heated at constant pressure to 320 K, then cooled at constant volume to its original temperature. How much heat flows into the gas during this two-step process?
A) 710 cal
B) -720 cal
C) 0.00 cal
D) 120 cal
E) -120 cal
A) 710 cal
B) -720 cal
C) 0.00 cal
D) 120 cal
E) -120 cal

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
27
A compression, at a constant pressure of 190 kPa, is performed on 5.0 moles of an ideal monatomic gas. The compression reduces the volume of the gas from 0.19 m3 to 0.12 m3. The ideal gas constant is R = 8.314 J/mol ∙ K. The work done by the gas is closest to
A) -13 kJ.
B) 13 kJ.
C) -33 kJ.
D) 33 kJ.
E) 0.00 kJ.
A) -13 kJ.
B) 13 kJ.
C) -33 kJ.
D) 33 kJ.
E) 0.00 kJ.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
28
An adiabatic compression is performed on an ideal gas. The final pressure is equal to 0.560 times the initial pressure and the final volume equals 1.50 times the initial volume. What is the adiabatic constant for the gas?
A) 1.33
B) 1.43
C) 1.48
D) 1.52
E) 1.67
A) 1.33
B) 1.43
C) 1.48
D) 1.52
E) 1.67

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
29
An expansion process on an ideal diatomic gas has a linear path between the initial and final states on a pV diagram. The initial pressure is 300 kPa, the initial volume is 0.060 m3, and the initial temperature is 390 K. The ideal gas constant is R = 8.314 J/mol ∙ K. The final pressure is 150 kPa and the final temperature is 260K. The work done by the gas is closest to
A) 4500 J.
B) 2300 J.
C) 3400 J.
D) 5600 J.
E) 6800 J.
A) 4500 J.
B) 2300 J.
C) 3400 J.
D) 5600 J.
E) 6800 J.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
30
A monatomic ideal gas undergoes an isothermal expansion at 300 K, as the volume increased from 0.020 m3 to 0.040 m3. The final pressure is 120 kPA. The ideal gas constant is R = 8.314 J/mol ∙ K. The heat transfer to the gas is closest to
A) 3.3 kJ.
B) 1.7 kJ.
C) -3.3 kJ.
D) -1.7 kJ.
E) 0.00 kJ.
A) 3.3 kJ.
B) 1.7 kJ.
C) -3.3 kJ.
D) -1.7 kJ.
E) 0.00 kJ.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
31
A certain amount of ideal monatomic gas is maintained at constant volume as it is cooled from 455K to 405 K. This feat is accomplished by removing 400 J of heat from the gas. How much work is done by the gas during this process? The ideal gas constant is R = 8.314 J/mol ∙ K.
A) 0.00 J
B) 200 J
C) 400 J
D) -400 J
E) -200 J
A) 0.00 J
B) 200 J
C) 400 J
D) -400 J
E) -200 J

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
32
The gas in a perfectly insulated system does work at a rate of 13 W. At what rate is the internal (thermal) energy of the gas changing?
A) -13 W
B) 13 W
C) 0.00 W
D) 6.5 W
A) -13 W
B) 13 W
C) 0.00 W
D) 6.5 W

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
33
An ideal gas with γ = 1.67 is initially at 0°C in a volume of 10.0 L at a pressure of 1.00 atm. It is then expanded adiabatically to a volume of 10.4 L. What is the final temperature of the gas?
A) -7.1°C
B) 2.5°C
C) -23°C
D) 68°C
E) -20°C
A) -7.1°C
B) 2.5°C
C) -23°C
D) 68°C
E) -20°C

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
34
A monatomic ideal gas undergoes an isothermal expansion at 300 K, as the volume increased from 0.03 m3 to 0.21 m3. The final pressure of the gas is 60 kPA. The ideal gas constant is R = 8.314 J/mol ∙ K. The change in the internal (thermal) energy of the gas is closest to
A) 0.00 kJ.
B) 12 kJ.
C) 25 kJ.
D) -12 kJ.
E) -25 kJ.
A) 0.00 kJ.
B) 12 kJ.
C) 25 kJ.
D) -12 kJ.
E) -25 kJ.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
35
During an isothermal process, 5.0 J of heat is removed from an ideal gas. What is the change in internal (thermal) energy of the gas?
A) 0.00 J
B) 2.5 J
C) 5.0 J
D) 7.5 J
E) 10 J
A) 0.00 J
B) 2.5 J
C) 5.0 J
D) 7.5 J
E) 10 J

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
36
A quantity of ideal gas requires 800 kJ to raise the temperature of the gas by 10.0 K when the gas is maintained at constant volume. The same quantity of gas requires 900 kJ to raise the temperature of the gas by 10.0 K when the gas is maintained at constant pressure. What is the adiabatic constant γ for this gas?
A) 0.889
B) 1.13
C) 1.22
D) 1.67
E) 1.40
A) 0.889
B) 1.13
C) 1.22
D) 1.67
E) 1.40

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
37
An ideal monatomic gas cools from 455.0 K to 405.0 K at constant volume as 831 J of energy is removed from it. How many moles of gas are in the sample? The ideal gas constant is R = 8.314 J/mol ∙ K.
A) 2.50 mol
B) 2.15 mol
C) 1.50 mol
D) 1.33 mol
E) 0.725 mol
A) 2.50 mol
B) 2.15 mol
C) 1.50 mol
D) 1.33 mol
E) 0.725 mol

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
38
A system has a heat source supplying heat to an ideal gas at a rate of 187.0 W and the gas is doing work at a rate of 130.9 W. At what rate is the internal (thermal) energy of the gas changing?
A) 56.1 W
B) 318 W
C) -56.1 W
D) 187 W
A) 56.1 W
B) 318 W
C) -56.1 W
D) 187 W

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
39
The temperature of an ideal gas in a sealed 0.40-m3 rigid container is reduced from 350 K to 270k The final pressure of the gas is 60 kPA. The molar heat capacity at constant volume of the gas is 28.0 J/mol · K. The heat absorbed by the gas is closest to
A) -24 kJ.
B) -31 kJ.
C) 24 kJ.
D) 31 kJ.
E) 0.00 kJ.
A) -24 kJ.
B) -31 kJ.
C) 24 kJ.
D) 31 kJ.
E) 0.00 kJ.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
40
During an adiabatic process, an ideal gas does 25 J of work. What is the change in the internal (thermal) energy of the gas during this process?
A) 0.00 J
B) 50 J
C) 25 J
D) -25 J
E) -50 J
A) 0.00 J
B) 50 J
C) 25 J
D) -25 J
E) -50 J

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
41
An ideal gas is allowed to expand slowly at constant temperature to twice its original volume. During the expansion, the gas absorbs 200 kJ of heat.
(a) What is the change in the internal (thermal) energy of the gas during the expansion?
(b) How much work does the gas do during the expansion?
(a) What is the change in the internal (thermal) energy of the gas during the expansion?
(b) How much work does the gas do during the expansion?

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
42
The figure shows the pV diagram for a certain thermodynamic process. In this process, 1500 J of heat flows into a system, and at the same time the system expands against a constant external pressure of 9.00 × 104 Pa. If the volume of the system increases from 0.020 m3 to 0.050 m3, calculate the change in internal (thermal) energy of the system. If the internal (thermal) energy change is nonzero, be sure to indicate whether this energy change is positive or negative. 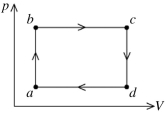


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
43
A cylinder contains 1.2 moles of ideal gas, initially at a temperature of 116°C. The cylinder is provided with a frictionless piston, which maintains a constant pressure of 6.4 × 105 Pa on the gas. The gas is cooled until its temperature has decreased to 27°C. For the gas CV = 11.65 J/mol ∙ K, and the ideal gas constant R = 8.314 J/mol ∙ K.
(a) Find the work done by (or on) the gas during this process. Is the work done by or on the gas?
(b) What is the change in the internal (thermal) energy of the gas during this process?
(c) How much heat is transferred to (or from) the gas during this process? Does this heat flow into or out of the gas?
(a) Find the work done by (or on) the gas during this process. Is the work done by or on the gas?
(b) What is the change in the internal (thermal) energy of the gas during this process?
(c) How much heat is transferred to (or from) the gas during this process? Does this heat flow into or out of the gas?

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
44
An ideal gas initially at 300 K and occupying a volume of 20 L is adiabatically compressed. If its final temperature is 400 K and γ = 1.30, what is its final volume?
A) 7.7 L
B) 14 L
C) 22 L
D) 52 L
A) 7.7 L
B) 14 L
C) 22 L
D) 52 L

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
45
During an adiabatic process, 20 moles of a monatomic ideal gas undergo a temperature change from 450 K to 320 K starting from an initial pressure is 400 kPa. The ideal gas constant is R = 8.314 J/mol ∙ K.
(a) What is the final volume of the gas?
(b) How much heat does the gas exchange during this process?
(c) What is the change in the internal (thermal) energy of the gas during this process?
(a) What is the final volume of the gas?
(b) How much heat does the gas exchange during this process?
(c) What is the change in the internal (thermal) energy of the gas during this process?

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
46
An ideal gas with γ = 1.30 occupies 7.0 L at 300 K and 200 kPa pressure. It is compressed adiabatically to 1/7 of its original volume, then cooled at constant volume to 300 K, and finally allowed to expand isothermally to 7.0 L. How much work does the gas do during this process? The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K.
A) -980 J
B) 6400 J
C) -270,000 J
D) -6400 J
E) 980 J
A) -980 J
B) 6400 J
C) -270,000 J
D) -6400 J
E) 980 J

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
47
A fixed amount of ideal gas goes through a process abc. In state a, the temperature of the gas is 152°C, its pressure is 1.25 atm, and it occupies a volume of 0.250 m3. It then undergoes an isothermal expansion to state b that doubles its volume, followed by an isobaric compression back to its original volume at state c. (Hint: First show this process on a pV diagram.) The ideal gas constant is 8.314 J/mol ∙ K, and 1.00 atm = 1.01 × 105 Pa.
(a) How many moles does this gas contain?
(b) What is the change in the internal energy of the gas between states a and b?
(c) What is the net work done on (or by) this gas during the entire process?
(d) What is the temperature of the gas in state c?
(a) How many moles does this gas contain?
(b) What is the change in the internal energy of the gas between states a and b?
(c) What is the net work done on (or by) this gas during the entire process?
(d) What is the temperature of the gas in state c?

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
48
A container with rigid walls is filled with 4 mol of air with CV = 2.5R. How much does the internal (thermal) energy change if the temperature of the air rises from 16°C to 437°C? The ideal gas constant is R = 8.314 J/mol ∙ K.
A) 35 kJ
B) 421 J
C) 3.5 kJ
D) 8.75 kJ
A) 35 kJ
B) 421 J
C) 3.5 kJ
D) 8.75 kJ

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
49
A cylinder contains 23 moles of an ideal gas at a temperature of 300 K. The gas is compressed at constant pressure until the final volume equals 0.43 times the initial volume. The molar heat capacity at constant volume of the gas is 24.0 J/mol ∙ K and the ideal gas constant is R = 8.314 J/mol ∙ K. The heat absorbed by the gas is closest to
A) -130 kJ.
B) -94 kJ.
C) 130 kJ.
D) 94 kJ.
E) -33 kJ.
A) -130 kJ.
B) -94 kJ.
C) 130 kJ.
D) 94 kJ.
E) -33 kJ.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
50
The pV diagram shown is for 7.50 moles of an ideal diatomic gas taken through a cycle from a to b to c. The ideal gas constant is R = 8.314 J/mol ∙ K. 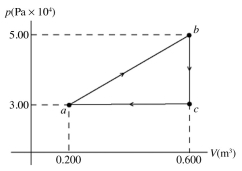
(a) What is the highest temperature reached by the gas during the cycle?
(b) What net work does the gas do during the cycle?
(c) How much heat is exchanged with the gas during part bc of the cycle? Does it enter or leave the gas?
(d) What is the change in the internal (thermal) energy of the gas during part bc of the cycle?
(e) What is the change in the internal (thermal) energy of the gas during the entire cycle?

(a) What is the highest temperature reached by the gas during the cycle?
(b) What net work does the gas do during the cycle?
(c) How much heat is exchanged with the gas during part bc of the cycle? Does it enter or leave the gas?
(d) What is the change in the internal (thermal) energy of the gas during part bc of the cycle?
(e) What is the change in the internal (thermal) energy of the gas during the entire cycle?

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
51
A cylinder contains 24.0 moles of an ideal gas at a temperature of 300 K. The gas is compressed at constant pressure until the final volume equals 0.63 times the initial volume. The molar heat capacity at constant volume of the gas is 24.0 J/mol ∙ K and the ideal gas constant is R = 8.314 J/mol ∙ K. The change in the internal (thermal) energy of the gas is closest to
A) -64 kJ.
B) -86 kJ.
C) 64 kJ.
D) 86 kJ.
E) -22 kJ.
A) -64 kJ.
B) -86 kJ.
C) 64 kJ.
D) 86 kJ.
E) -22 kJ.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
52
In a thermodynamic process involving 7.8 moles of an ideal gas, the gas is at an initial temperature of 24°C and has an initial volume of 0.040 m3. The gas expands adiabatically to a volume of 0.080 m3. For this gas, CV = 12.27 J/mol · K, and the ideal gas constant is R = 8.314 J/mol ∙ K. Calculate the work done by the gas during this expansion.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck


