Deck 2: Water and Carbon: the Chemical Basis of Life
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/59
Play
Full screen (f)
Deck 2: Water and Carbon: the Chemical Basis of Life
1
Knowing the atomic mass of an element allows inferences about which of the following?
A) the number of electrons in the element
B) the number of protons in the element
C) the number of neutrons in the element
D) the number of protons plus neutrons in the element
E) the number of protons plus electrons in the element
A) the number of electrons in the element
B) the number of protons in the element
C) the number of neutrons in the element
D) the number of protons plus neutrons in the element
E) the number of protons plus electrons in the element
D
2
When the atoms involved in a covalent bond have the same electronegativity, what type of bond results?
A) an ionic bond
B) a hydrogen bond
C) a nonpolar covalent bond
D) a polar covalent bond
A) an ionic bond
B) a hydrogen bond
C) a nonpolar covalent bond
D) a polar covalent bond
C
3
Why is each element unique with respect to its chemical properties? Each element has a distinctive ________.
A) atomic mass
B) number of electrons
C) number of protons
D) number of neutrons
E) radioactive property
A) atomic mass
B) number of electrons
C) number of protons
D) number of neutrons
E) radioactive property
C
4
How many electrons are involved in a single covalent bond?
A) one
B) two
C) three
D) four
A) one
B) two
C) three
D) four

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
5
An atom has four electrons in its valence shell. What types of covalent bonds is it capable of forming?
A) single, double, or triple
B) single and double only
C) single bonds only
D) double bonds only
A) single, double, or triple
B) single and double only
C) single bonds only
D) double bonds only

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
6
A covalent bond is likely to be polar when ________.
A) one of the atoms sharing electrons is more electronegative than the other atom
B) the two atoms sharing electrons are equally electronegative
C) carbon is one of the two atoms sharing electrons
D) one of the atoms has absorbed more energy than the other atom
E) the two atoms sharing electrons are the same elements
A) one of the atoms sharing electrons is more electronegative than the other atom
B) the two atoms sharing electrons are equally electronegative
C) carbon is one of the two atoms sharing electrons
D) one of the atoms has absorbed more energy than the other atom
E) the two atoms sharing electrons are the same elements

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
7
Bonds between two atoms that are equally electronegative are ________.
A) hydrogen bonds
B) van der Waals interactions
C) polar covalent bonds
D) nonpolar covalent bonds
E) ionic bonds
A) hydrogen bonds
B) van der Waals interactions
C) polar covalent bonds
D) nonpolar covalent bonds
E) ionic bonds

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
8
Refer to the following figure to answer the questions below. 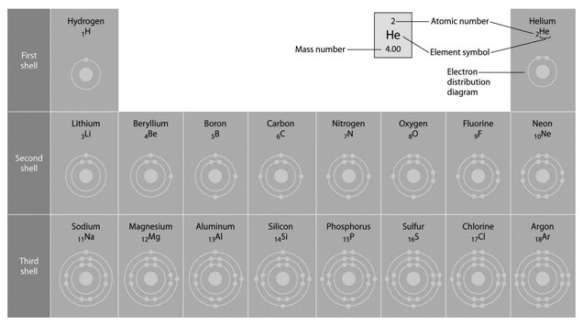 Refer to the figure above (first three rows of the periodic table). What element has properties most similar to carbon?
Refer to the figure above (first three rows of the periodic table). What element has properties most similar to carbon?
A) boron
B) silicon
C) nitrogen
D) aluminum
E) phosphorus
 Refer to the figure above (first three rows of the periodic table). What element has properties most similar to carbon?
Refer to the figure above (first three rows of the periodic table). What element has properties most similar to carbon?A) boron
B) silicon
C) nitrogen
D) aluminum
E) phosphorus

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
9
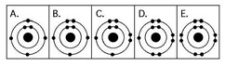
Which drawing in the figure above depicts an atom with six valence electrons?
A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
10
Nitrogen (N) is more electronegative than hydrogen (H). Which of the following is a correct statement about the atoms in ammonia (NH3)?
A) Each hydrogen atom has a partial positive charge; the nitrogen atom has a partial negative charge.
B) Ammonia has an overall positive charge.
C) Ammonia has an overall negative charge.
D) The nitrogen atom has a partial positive charge; each hydrogen atom has a partial negative charge.
E) There are covalent bonds between the hydrogen atoms and polar bonds between each hydrogen atom and the nitrogen atom.
A) Each hydrogen atom has a partial positive charge; the nitrogen atom has a partial negative charge.
B) Ammonia has an overall positive charge.
C) Ammonia has an overall negative charge.
D) The nitrogen atom has a partial positive charge; each hydrogen atom has a partial negative charge.
E) There are covalent bonds between the hydrogen atoms and polar bonds between each hydrogen atom and the nitrogen atom.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
11
Which one of the following pairs of neutral atoms would be most likely to form ions and thus an ionic bond?
A)
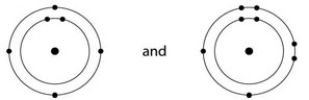
B)

C)
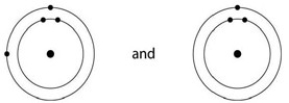
D)
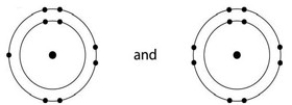
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
12

Which drawing in the figure above depicts the electron configuration of an element with chemical properties most similar to helium (2He)?
A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
13
Carbon-12 is the most common isotope of carbon and has a mass number of 12. However, the average atomic mass of carbon found on a periodic table is slightly more than 12 daltons. Why?
A) The atomic mass does not include the mass of electrons.
B) Some carbon atoms in nature have an extra proton.
C) Some carbon atoms in nature have more neutrons.
D) Some carbon atoms in nature have a different valence electron distribution.
E) Some carbon atoms in nature have undergone radioactive decay.
A) The atomic mass does not include the mass of electrons.
B) Some carbon atoms in nature have an extra proton.
C) Some carbon atoms in nature have more neutrons.
D) Some carbon atoms in nature have a different valence electron distribution.
E) Some carbon atoms in nature have undergone radioactive decay.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
14
About twenty-five of the 92 natural elements are known to be essential to life. Which 4 of these 25 elements make up approximately 96 percent of living matter?
A) carbon, sodium, hydrogen, nitrogen
B) carbon, oxygen, phosphorus, hydrogen
C) oxygen, hydrogen, calcium, nitrogen
D) carbon, hydrogen, nitrogen, oxygen
E) carbon, oxygen, nitrogen, calcium
A) carbon, sodium, hydrogen, nitrogen
B) carbon, oxygen, phosphorus, hydrogen
C) oxygen, hydrogen, calcium, nitrogen
D) carbon, hydrogen, nitrogen, oxygen
E) carbon, oxygen, nitrogen, calcium

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
15
What is the difference between covalent bonds and ionic bonds?
A) Covalent bonds require carbon whereas ionic bonds do not.
B) Covalent bonds involve the sharing of pairs of electrons between atoms; ionic bonds involve the sharing of single electrons between atoms.
C) Covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the electrical attraction between charged atoms.
D) Covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the sharing of protons between charged atoms.
E) Covalent bonds involve the transfer of electrons between charged atoms; ionic bonds involve the sharing of electrons between atoms.
A) Covalent bonds require carbon whereas ionic bonds do not.
B) Covalent bonds involve the sharing of pairs of electrons between atoms; ionic bonds involve the sharing of single electrons between atoms.
C) Covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the electrical attraction between charged atoms.
D) Covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the sharing of protons between charged atoms.
E) Covalent bonds involve the transfer of electrons between charged atoms; ionic bonds involve the sharing of electrons between atoms.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
16
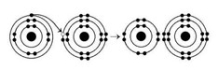 The illustration above shows a representation of formic acid. A formic acid molecule ________.
The illustration above shows a representation of formic acid. A formic acid molecule ________.A) will form hydrogen bonds with water molecules
B) has a tetrahedral configuration of hybrid electron orbitals for the carbon atom
C) consists of largely nonpolar covalent bonds
D) is held together by hydrogen bonds
E) has a pyramidal shape and will form hydrogen bonds with water molecules

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
17
Elements found on the left side of the periodic table contain outer shells that are ________; these elements tend to form ________ in solution.
A) almost empty; cations
B) almost empty; anions
C) almost full; cations
D) almost full; anions
A) almost empty; cations
B) almost empty; anions
C) almost full; cations
D) almost full; anions

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
18
From its atomic number of 15, it is possible to predict that the phosphorus atom has ________.
A) 5 neutrons, 5 protons, and 5 electrons
B) 30 neutrons
C) 15 neutrons and 15 protons
D) 8 electrons in its outermost electron shell
E) 15 protons and 15 electrons
A) 5 neutrons, 5 protons, and 5 electrons
B) 30 neutrons
C) 15 neutrons and 15 protons
D) 8 electrons in its outermost electron shell
E) 15 protons and 15 electrons

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
19
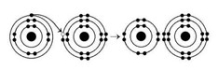 What results from the chemical reaction illustrated above? The reactants have no charge.
What results from the chemical reaction illustrated above? The reactants have no charge.A) a cation with a net charge of +1 and an anion with a net charge of +1
B) a cation with a net charge of −1 and an anion with a net charge of −1
C) a cation with a net charge of −1 and an anion with a net charge of +1
D) a cation with a net charge of +1 and an anion with a net charge of −1

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
20
A covalent chemical bond is one in which ________.
A) electrons are removed from one atom and transferred to another atom so that the two atoms become oppositely charged
B) protons and neutrons are shared by two atoms so as to satisfy the requirements of both atoms
C) outer-shell electrons of two atoms are shared so as to satisfactorily fill their respective orbitals
D) outer-shell electrons of one atom are transferred to fill the inner electron shell of another atom
E) electrons from the same atom, but opposite spins, are paired
A) electrons are removed from one atom and transferred to another atom so that the two atoms become oppositely charged
B) protons and neutrons are shared by two atoms so as to satisfy the requirements of both atoms
C) outer-shell electrons of two atoms are shared so as to satisfactorily fill their respective orbitals
D) outer-shell electrons of one atom are transferred to fill the inner electron shell of another atom
E) electrons from the same atom, but opposite spins, are paired

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
21
The partial negative charge in a molecule of water occurs because ________.
A) the oxygen atom donates an electron to each of the hydrogen atoms
B) the electrons shared between the oxygen and hydrogen atoms spend more time around the oxygen atom nucleus than around the hydrogen atom nucleus
C) the oxygen atom has two pairs of electrons in its valence shell that are not neutralized by hydrogen atoms
D) the oxygen atom forms hybrid orbitals that distribute electrons unequally around the oxygen nucleus
E) one of the hydrogen atoms donates an electron to the oxygen atom
A) the oxygen atom donates an electron to each of the hydrogen atoms
B) the electrons shared between the oxygen and hydrogen atoms spend more time around the oxygen atom nucleus than around the hydrogen atom nucleus
C) the oxygen atom has two pairs of electrons in its valence shell that are not neutralized by hydrogen atoms
D) the oxygen atom forms hybrid orbitals that distribute electrons unequally around the oxygen nucleus
E) one of the hydrogen atoms donates an electron to the oxygen atom

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
22
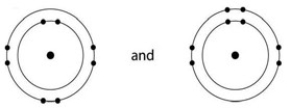
Based on your knowledge of the polarity of water molecules, the solute molecule depicted here is most likely ________. 
A) positively charged
B) negatively charged
C) without charge
D) hydrophobic
E) nonpolar

A) positively charged
B) negatively charged
C) without charge
D) hydrophobic
E) nonpolar

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
23
You need to represent a molecule to best illustrate the relative sizes of the atoms involved and their interrelationships. Which representation would work best?
A) molecular formula
B) structural formula
C) ball-and-stick model
D) space-filling model
A) molecular formula
B) structural formula
C) ball-and-stick model
D) space-filling model

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
24
You have two beakers. One contains pure water; the other contains pure methanol (wood alcohol). The covalent bonds of methanol molecules are nonpolar, so there are no hydrogen bonds among methanol molecules. You pour crystals of table salt (NaCl) into each beaker. Predict what will happen.
A) Equal amounts of NaCl crystals will dissolve in both water and methanol.
B) NaCl crystals will not dissolve in either water or methanol.
C) NaCl crystals will dissolve readily in water but will not dissolve in methanol.
D) NaCl crystals will dissolve readily in methanol but will not dissolve in water.
E) When the first crystals of NaCl are added to water or to methanol, they will not dissolve; but as more crystals are added, the crystals will begin to dissolve faster and faster.
A) Equal amounts of NaCl crystals will dissolve in both water and methanol.
B) NaCl crystals will not dissolve in either water or methanol.
C) NaCl crystals will dissolve readily in water but will not dissolve in methanol.
D) NaCl crystals will dissolve readily in methanol but will not dissolve in water.
E) When the first crystals of NaCl are added to water or to methanol, they will not dissolve; but as more crystals are added, the crystals will begin to dissolve faster and faster.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
25
One of the buffers that contribute to pH stability in human blood is carbonic acid (H2CO3). Carbonic acid is a weak acid that, when placed in an aqueous solution, dissociates into a bicarbonate ion (HCO3−) and a hydrogen ion (H+), as noted below. 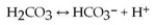 If the pH of blood increases, one would expect ________.
If the pH of blood increases, one would expect ________.
A) a decrease in the concentration of H2CO3 and an increase in the concentration of HCO3−
B) an increase in the concentration of H2CO3 and a decrease in the concentration of HCO3−
C) a decrease in the concentration of HCO3− and an increase in the concentration of H+
D) an increase in the concentration of HCO3− and a decrease in the concentration of OH−
E) a decrease in the concentration of HCO3− and an increase in the concentration of H2CO3 and H+
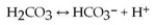 If the pH of blood increases, one would expect ________.
If the pH of blood increases, one would expect ________.A) a decrease in the concentration of H2CO3 and an increase in the concentration of HCO3−
B) an increase in the concentration of H2CO3 and a decrease in the concentration of HCO3−
C) a decrease in the concentration of HCO3− and an increase in the concentration of H+
D) an increase in the concentration of HCO3− and a decrease in the concentration of OH−
E) a decrease in the concentration of HCO3− and an increase in the concentration of H2CO3 and H+

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
26
Why does ice float in liquid water?
A) The high surface tension of liquid water keeps the ice on top.
B) The ionic bonds between the molecules in ice prevent the ice from sinking.
C) Ice always has air bubbles that keep it afloat.
D) Stable hydrogen bonds keep water molecules of ice farther apart than water molecules of liquid water.
E) The crystalline lattice of ice causes it to be denser than liquid water.
A) The high surface tension of liquid water keeps the ice on top.
B) The ionic bonds between the molecules in ice prevent the ice from sinking.
C) Ice always has air bubbles that keep it afloat.
D) Stable hydrogen bonds keep water molecules of ice farther apart than water molecules of liquid water.
E) The crystalline lattice of ice causes it to be denser than liquid water.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
27
The partial negative charge at one end of a water molecule is attracted to the partial positive charge of another water molecule. What is this attraction called?
A) a covalent bond
B) a hydrogen bond
C) an ionic bond
D) a hydrophilic bond
E) a van der Waals interaction
A) a covalent bond
B) a hydrogen bond
C) an ionic bond
D) a hydrophilic bond
E) a van der Waals interaction

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
28
In a single molecule of water, two hydrogen atoms are bonded to a single oxygen atom by ________.
A) hydrogen bonds
B) nonpolar covalent bonds
C) polar covalent bonds
D) ionic bonds
E) van der Waals interactions
A) hydrogen bonds
B) nonpolar covalent bonds
C) polar covalent bonds
D) ionic bonds
E) van der Waals interactions

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
29
Which type of bond must be broken for water to vaporize?
A) ionic bonds
B) both hydrogen bonds and ionic bonds
C) polar covalent bonds
D) hydrogen bonds
E) both polar covalent bonds and hydrogen bonds
A) ionic bonds
B) both hydrogen bonds and ionic bonds
C) polar covalent bonds
D) hydrogen bonds
E) both polar covalent bonds and hydrogen bonds

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following is a property of liquid water? Liquid water ________.
A) is less dense than ice
B) has a specific heat that is lower than that for most other substances
C) has a heat of vaporization that is higher than that for most other substances
D) is nonpolar
A) is less dense than ice
B) has a specific heat that is lower than that for most other substances
C) has a heat of vaporization that is higher than that for most other substances
D) is nonpolar

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
31
A solution contains 0.0000001 (10−7) moles of hydroxyl ions [OH−] per liter. Which of the following best describes this solution?
A) acidic: H+ acceptor
B) basic: H+ acceptor
C) acidic: H+ donor
D) basic: H+ donor
E) neutral
A) acidic: H+ acceptor
B) basic: H+ acceptor
C) acidic: H+ donor
D) basic: H+ donor
E) neutral

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
32
Water has a high specific heat because of the ________.
A) polar covalent bond formed between the oxygen and a hydrogen of a single water molecule
B) ionic bonds formed between the hydrogen of one water molecule and the oxygen of another water molecule
C) hydrogen bond formed between the hydrogen of one water molecule and the oxygen of another water molecule
D) covalent bond formed between the hydrogen of one water molecule and the oxygen of another water molecule
A) polar covalent bond formed between the oxygen and a hydrogen of a single water molecule
B) ionic bonds formed between the hydrogen of one water molecule and the oxygen of another water molecule
C) hydrogen bond formed between the hydrogen of one water molecule and the oxygen of another water molecule
D) covalent bond formed between the hydrogen of one water molecule and the oxygen of another water molecule

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following takes place as an ice cube cools a drink?
A) Molecular collisions in the drink increase.
B) Kinetic energy in the liquid water decreases.
C) A calorie of heat energy is transferred from the ice to the water of the drink.
D) The specific heat of the water in the drink decreases.
E) Evaporation of the water in the drink increases.
A) Molecular collisions in the drink increase.
B) Kinetic energy in the liquid water decreases.
C) A calorie of heat energy is transferred from the ice to the water of the drink.
D) The specific heat of the water in the drink decreases.
E) Evaporation of the water in the drink increases.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
34
Why are hydrocarbons insoluble in water?
A) The majority of their bonds are polar covalent carbon-to-hydrogen linkages.
B) The majority of their bonds are nonpolar covalent carbon-to-hydrogen linkages.
C) They are hydrophilic.
D) They exhibit considerable molecular complexity and diversity.
E) They are less dense than water.
A) The majority of their bonds are polar covalent carbon-to-hydrogen linkages.
B) The majority of their bonds are nonpolar covalent carbon-to-hydrogen linkages.
C) They are hydrophilic.
D) They exhibit considerable molecular complexity and diversity.
E) They are less dense than water.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
35
To act as an effective coolant in a car's radiator, a substance has to have the capacity to absorb a great deal of heat. You have a reference book with tables listing the physical properties of many liquids. In choosing a coolant for your car, which table would you check first?
A) pH
B) density at room temperature
C) heat of vaporization
D) specific heat
A) pH
B) density at room temperature
C) heat of vaporization
D) specific heat

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
36
A solution with a pH of 5 has how many more protons in it than a solution with a pH of 7?
A) 5 times
B) 10 times
C) 100 times
D) 1000 times
A) 5 times
B) 10 times
C) 100 times
D) 1000 times

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
37
A strong acid like HCl ________.
A) dissociates completely in an aqueous solution
B) increases the pH when added to an aqueous solution
C) reacts with strong bases to create a buffered solution
D) is a strong buffer at low pH
E) dissociates completely in aqueous solutions and is a strong buffer at low pH
A) dissociates completely in an aqueous solution
B) increases the pH when added to an aqueous solution
C) reacts with strong bases to create a buffered solution
D) is a strong buffer at low pH
E) dissociates completely in aqueous solutions and is a strong buffer at low pH

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following effects can occur because of the high surface tension of water?
A) Lakes cannot freeze solid in winter, despite low temperatures.
B) A raft spider can walk across the surface of a small pond.
C) Organisms can resist temperature changes, although they give off heat due to chemical reactions.
D) Sweat can evaporate from the skin, helping to keep people from overheating.
E) Water can flow upward from roots to the leaves in plants.
A) Lakes cannot freeze solid in winter, despite low temperatures.
B) A raft spider can walk across the surface of a small pond.
C) Organisms can resist temperature changes, although they give off heat due to chemical reactions.
D) Sweat can evaporate from the skin, helping to keep people from overheating.
E) Water can flow upward from roots to the leaves in plants.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
39
What is the pH of a solution with a hydroxyl ion (OH−) concentration of 10−12 M?
A) pH 2
B) pH 4
C) pH 10
D) pH 12
E) pH 14
A) pH 2
B) pH 4
C) pH 10
D) pH 12
E) pH 14

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
40
You need to write down information about a molecule, but need to indicate only the type and number of atoms it contains. Which representation would work best?
A) molecular formula
B) structural formula
C) ball-and-stick model
D) space-filling model
A) molecular formula
B) structural formula
C) ball-and-stick model
D) space-filling model

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following is the most spontaneous reaction? A reaction that is ________.
A) slightly exothermic and leads to a slight increase in entropy
B) slightly endothermic and leads to a huge decrease in entropy
C) highly exothermic and leads to a huge decrease in entropy
D) slightly exothermic and leads to a huge increase in entropy
A) slightly exothermic and leads to a slight increase in entropy
B) slightly endothermic and leads to a huge decrease in entropy
C) highly exothermic and leads to a huge decrease in entropy
D) slightly exothermic and leads to a huge increase in entropy

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following correctly describes all chemical equilibrium?
A) Forward and reverse reactions continue with no net effect on the concentrations of the reactants and products.
B) Concentrations of products are higher than the concentrations of the reactants.
C) There are equal concentrations of products and reactants while forward and reverse reactions continue.
D) Reactions stop only when all reactants have been converted to products.
E) There are equal concentrations of reactants and products, and the reactions have stopped.
A) Forward and reverse reactions continue with no net effect on the concentrations of the reactants and products.
B) Concentrations of products are higher than the concentrations of the reactants.
C) There are equal concentrations of products and reactants while forward and reverse reactions continue.
D) Reactions stop only when all reactants have been converted to products.
E) There are equal concentrations of reactants and products, and the reactions have stopped.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following always tends to make chemical reactions spontaneous?
A) The reactants have lower potential energy than the products.
B) The reactants are more ordered than the products.
C) The temperature is low.
D) The pressure is low.
A) The reactants have lower potential energy than the products.
B) The reactants are more ordered than the products.
C) The temperature is low.
D) The pressure is low.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
44
What does it mean to say a system's energy is equal to zero?
A) The system does not release or absorb heat.
B) The system is perfectly ordered (no entropy).
C) The total amount of potential energy in the system is zero.
D) The system is at equilibrium.
A) The system does not release or absorb heat.
B) The system is perfectly ordered (no entropy).
C) The total amount of potential energy in the system is zero.
D) The system is at equilibrium.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following is TRUE for this reaction? 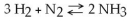
A) The reaction is nonreversible.
B) Hydrogen and nitrogen are the reactants of the reverse reaction.
C) Hydrogen and nitrogen are the products of the forward reaction.
D) Ammonia is being formed and decomposed simultaneously.
E) Only the forward or reverse reactions can occur at one time.
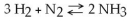
A) The reaction is nonreversible.
B) Hydrogen and nitrogen are the reactants of the reverse reaction.
C) Hydrogen and nitrogen are the products of the forward reaction.
D) Ammonia is being formed and decomposed simultaneously.
E) Only the forward or reverse reactions can occur at one time.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
46
The complexity and variety of organic molecules is due to ________.
A) the chemical versatility of carbon atoms
B) the variety of rare elements in organic molecules
C) the diverse bonding patterns of nitrogen
D) their interaction with water
E) their tremendously large sizes
A) the chemical versatility of carbon atoms
B) the variety of rare elements in organic molecules
C) the diverse bonding patterns of nitrogen
D) their interaction with water
E) their tremendously large sizes

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
47
A carbon atom is most likely to form what kind of bond(s) with other atoms?
A) ionic
B) hydrogen
C) covalent
D) covalent bonds and hydrogen bonds
E) ionic bonds, covalent bonds, and hydrogen bonds
A) ionic
B) hydrogen
C) covalent
D) covalent bonds and hydrogen bonds
E) ionic bonds, covalent bonds, and hydrogen bonds

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
48
Ice melts spontaneously at room temperature, even though the process is endothermic. How is this possible?
A) The change in thermal energy is small, so melting still obeys the second law of thermodynamics.
B) The first law of thermodynamics does not apply to phase changes such as melting.
C) Water has a very high specific heat.
D) There is a large increase in entropy.
A) The change in thermal energy is small, so melting still obeys the second law of thermodynamics.
B) The first law of thermodynamics does not apply to phase changes such as melting.
C) Water has a very high specific heat.
D) There is a large increase in entropy.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following correctly describes a reaction that has reached chemical equilibrium?
A) The concentration of the reactants equals the concentration of the products.
B) The rate of the forward reaction is equal to the rate of the reverse reaction.
C) All of the reactants have been converted to the products of the reaction.
D) All of the products have been converted to the reactants of the reaction.
E) Both the forward and the reverse reactions have stopped, with no net effect on the concentration of the reactants and the products.
A) The concentration of the reactants equals the concentration of the products.
B) The rate of the forward reaction is equal to the rate of the reverse reaction.
C) All of the reactants have been converted to the products of the reaction.
D) All of the products have been converted to the reactants of the reaction.
E) Both the forward and the reverse reactions have stopped, with no net effect on the concentration of the reactants and the products.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
50
During chemical evolution, which of the following occurred in the molecules involved?
A) Entropy decreased while potential energy increased.
B) Entropy increased while potential energy increased.
C) Entropy stayed constant while potential energy increased.
A) Entropy decreased while potential energy increased.
B) Entropy increased while potential energy increased.
C) Entropy stayed constant while potential energy increased.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
51
Why is carbon so important in biology?
A) It is a common element on Earth.
B) It has very little electronegativity, making it a good electron donor.
C) It bonds to only a few other elements.
D) It can form a variety of carbon skeletons and host functional groups.
A) It is a common element on Earth.
B) It has very little electronegativity, making it a good electron donor.
C) It bonds to only a few other elements.
D) It can form a variety of carbon skeletons and host functional groups.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the functional groups below acts most like an acid in water?
A) amino
B) carbonyl
C) carboxyl
D) hydroxyl
A) amino
B) carbonyl
C) carboxyl
D) hydroxyl

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
53
Why are some reactions exothermic?
A) The products have lower potential energy than the reactants.
B) They are spontaneous.
C) They are not spontaneous.
D) The products have higher entropy than the reactants.
A) The products have lower potential energy than the reactants.
B) They are spontaneous.
C) They are not spontaneous.
D) The products have higher entropy than the reactants.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following is TRUE of carbon?
A) It forms only polar molecules.
B) It can form a maximum of three covalent bonds with other elements.
C) It is highly electronegative.
D) It can form both polar and nonpolar bonds.
A) It forms only polar molecules.
B) It can form a maximum of three covalent bonds with other elements.
C) It is highly electronegative.
D) It can form both polar and nonpolar bonds.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
55
Why do chemical reactions tend to speed up when the concentration of the reactants is increased?
A) The reactants move faster.
B) The reactants collide more often.
C) The reactants have greater energy.
D) All of the listed responses are correct.
A) The reactants move faster.
B) The reactants collide more often.
C) The reactants have greater energy.
D) All of the listed responses are correct.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
56
The first chemicals that provided potential energy on Earth may have been formaldehyde and hydrogen cyanide. While these were produced by sunlight-driven reactions, they also occurred around deep-sea vents. If the first organisms on Earth evolved around these vents, the first life on Earth was ________.
A) photosynthetic, obtaining energy from the Sun
B) chemosynthetic, obtaining energy from chemicals
C) herbivorous, obtaining energy from plants
D) carnivorous, obtaining energy from animals
A) photosynthetic, obtaining energy from the Sun
B) chemosynthetic, obtaining energy from chemicals
C) herbivorous, obtaining energy from plants
D) carnivorous, obtaining energy from animals

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
57
________ atoms give organic molecules their overall shape; ________ atoms determine the overall chemical behavior of organic molecules.
A) Carbon; H, N, and O
B) Hydrogen; C, N, and O
C) Carbon; H2O
D) H, N, and O; carbon
A) Carbon; H, N, and O
B) Hydrogen; C, N, and O
C) Carbon; H2O
D) H, N, and O; carbon

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
58
Stanley Miller's 1953 experiments supported the hypothesis that ________.
A) life on Earth arose from simple inorganic molecules
B) organic molecules can be synthesized abiotically under conditions that may have existed on early Earth
C) life on Earth arose from simple organic molecules, with energy from lightning and volcanoes
D) the conditions on early Earth were conducive to the origin of life
E) the conditions on early Earth were conducive to the abiotic synthesis of organic molecules
A) life on Earth arose from simple inorganic molecules
B) organic molecules can be synthesized abiotically under conditions that may have existed on early Earth
C) life on Earth arose from simple organic molecules, with energy from lightning and volcanoes
D) the conditions on early Earth were conducive to the origin of life
E) the conditions on early Earth were conducive to the abiotic synthesis of organic molecules

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
59
Consider the following reaction at equilibrium: 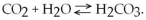 What would be the effect of adding additional H2CO3?
What would be the effect of adding additional H2CO3?
A) It would drive the equilibrium dynamics to the right.
B) It would drive the equilibrium dynamics to the left.
C) Nothing would happen, because the reactants and products are in equilibrium.
D) The amounts of CO2 and H2O would decrease.
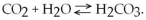 What would be the effect of adding additional H2CO3?
What would be the effect of adding additional H2CO3?A) It would drive the equilibrium dynamics to the right.
B) It would drive the equilibrium dynamics to the left.
C) Nothing would happen, because the reactants and products are in equilibrium.
D) The amounts of CO2 and H2O would decrease.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck


