Exam 2: Water and Carbon: the Chemical Basis of Life
Exam 1: Biology and the Tree of Life37 Questions
Exam 2: Water and Carbon: the Chemical Basis of Life59 Questions
Exam 3: Protein Structure and Function59 Questions
Exam 4: Nucleic Acids and the Rna World43 Questions
Exam 5: An Introduction to Carbohydrates44 Questions
Exam 53: Ecosystems and Global Ecology57 Questions
Exam 6: Lipids, Membranes, and the First Cells59 Questions
Exam 7: Inside the Cell60 Questions
Exam 8: Energy and Enzymes: an Introduction to Metabolism60 Questions
Exam 9: Cellular Respiration and Fermentation61 Questions
Exam 10: Photosynthesis58 Questions
Exam 11: Cellcell Interactions52 Questions
Exam 12: The Cell Cycle59 Questions
Exam 13: Meiosis63 Questions
Exam 14: Mendel and the Gene60 Questions
Exam 15: Dna and the Gene: Synthesis and Repair51 Questions
Exam 16: How Genes Work48 Questions
Exam 17: Transcription, Rna Processing, and Translation58 Questions
Exam 18: Control of Gene Expression in Bacteria29 Questions
Exam 19: Control of Gene Expression in Eukaryotes56 Questions
Exam 20: The Molecular Revolution: Biotechnology and Beyond70 Questions
Exam 21: Genes, Development, and Evolution38 Questions
Exam 22: Evolution by Natural Selection38 Questions
Exam 23: Evolutionary Processes37 Questions
Exam 24: Speciation56 Questions
Exam 25: Phylogenies and the History of Life63 Questions
Exam 26: Bacteria and Archaea38 Questions
Exam 27: Protists37 Questions
Exam 28: Green Algae and Land Plants59 Questions
Exam 29: Fungi47 Questions
Exam 30: An Introduction to Animals48 Questions
Exam 31: Protostome Animals54 Questions
Exam 32: Deuterostome Animals60 Questions
Exam 33: Viruses44 Questions
Exam 34: Plant Form and Function46 Questions
Exam 35: Water and Sugar Transport in Plants47 Questions
Exam 36: Plant Nutrition54 Questions
Exam 37: Plant Sensory Systems, Signals, and Responses48 Questions
Exam 38: Plant Reproduction and Development51 Questions
Exam 39: Animal Form and Function53 Questions
Exam 40: Water and Electrolyte Balance in Animals60 Questions
Exam 41: Animal Nutrition94 Questions
Exam 42: Gas Exchange and Circulation93 Questions
Exam 43: Animal Nervous Systems100 Questions
Exam 44: Animal Sensory Systems50 Questions
Exam 45: Animal Movement40 Questions
Exam 46: Chemical Signals in Animals59 Questions
Exam 47: Animal Reproduction and Development104 Questions
Exam 48: The Immune System in Animals77 Questions
Exam 49: An Introduction to Ecology40 Questions
Exam 50: Behavioral Ecology40 Questions
Exam 51: Population Ecology57 Questions
Exam 52: Community Ecology55 Questions
Exam 54: Biodiversity and Conservation Biology43 Questions
Select questions type
You have two beakers. One contains pure water; the other contains pure methanol (wood alcohol). The covalent bonds of methanol molecules are nonpolar, so there are no hydrogen bonds among methanol molecules. You pour crystals of table salt (NaCl) into each beaker. Predict what will happen.
Free
(Multiple Choice)
4.7/5  (30)
(30)
Correct Answer:
C
Which of the following is TRUE of carbon?
Free
(Multiple Choice)
4.8/5  (41)
(41)
Correct Answer:
D
The complexity and variety of organic molecules is due to ________.
(Multiple Choice)
4.8/5  (26)
(26)
Which one of the following pairs of neutral atoms would be most likely to form ions and thus an ionic bond?
(Multiple Choice)
5.0/5  (36)
(36)
Refer to the following figure to answer the questions below. 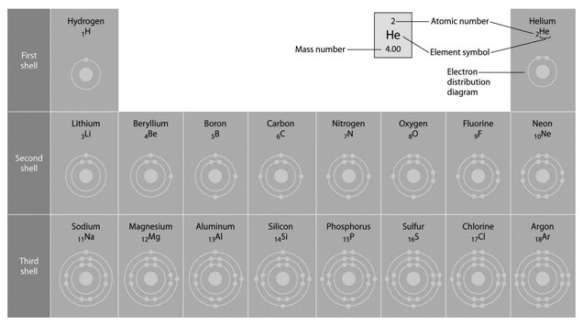 Refer to the figure above (first three rows of the periodic table). What element has properties most similar to carbon?
Refer to the figure above (first three rows of the periodic table). What element has properties most similar to carbon?
(Multiple Choice)
4.8/5  (20)
(20)
Knowing the atomic mass of an element allows inferences about which of the following?
(Multiple Choice)
4.9/5  (43)
(43)
The partial negative charge in a molecule of water occurs because ________.
(Multiple Choice)
4.9/5  (32)
(32)
What does it mean to say a system's energy is equal to zero?
(Multiple Choice)
4.9/5  (33)
(33)
You need to represent a molecule to best illustrate the relative sizes of the atoms involved and their interrelationships. Which representation would work best?
(Multiple Choice)
4.9/5  (36)
(36)
The first chemicals that provided potential energy on Earth may have been formaldehyde and hydrogen cyanide. While these were produced by sunlight-driven reactions, they also occurred around deep-sea vents. If the first organisms on Earth evolved around these vents, the first life on Earth was ________.
(Multiple Choice)
4.8/5  (31)
(31)
A carbon atom is most likely to form what kind of bond(s) with other atoms?
(Multiple Choice)
4.8/5  (42)
(42)
Carbon-12 is the most common isotope of carbon and has a mass number of 12. However, the average atomic mass of carbon found on a periodic table is slightly more than 12 daltons. Why?
(Multiple Choice)
4.9/5  (40)
(40)
A solution with a pH of 5 has how many more protons in it than a solution with a pH of 7?
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following correctly describes a reaction that has reached chemical equilibrium?
(Multiple Choice)
4.9/5  (37)
(37)
Why do chemical reactions tend to speed up when the concentration of the reactants is increased?
(Multiple Choice)
4.9/5  (39)
(39)
Showing 1 - 20 of 59
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)