Deck 3: An Introduction to Organic Reactions: Acids and Bases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/47
Play
Full screen (f)
Deck 3: An Introduction to Organic Reactions: Acids and Bases
1
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 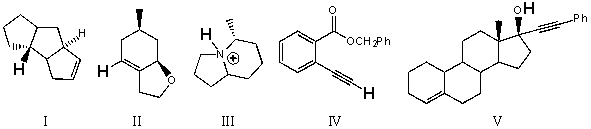
A) III > V > IV > II > I
B) V > IV > III > II > I
C) V > III > IV > II > I
D) III > V > II > IV > I
E) IV > II > I > V > III

A) III > V > IV > II > I
B) V > IV > III > II > I
C) V > III > IV > II > I
D) III > V > II > IV > I
E) IV > II > I > V > III
III > V > IV > II > I
2
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 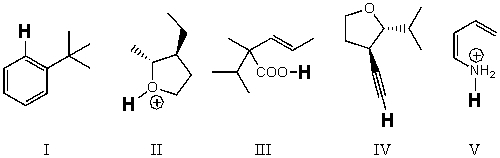
A) III > II > IV > V > I
B) II > III > V > IV > I
C) II > V > III > IV > I
D) V > III > II > IV > I
E) I > IV > II > III > V

A) III > II > IV > V > I
B) II > III > V > IV > I
C) II > V > III > IV > I
D) V > III > II > IV > I
E) I > IV > II > III > V
II > III > V > IV > I
3
What is/are the product(s)of the following acid-base mechanism? 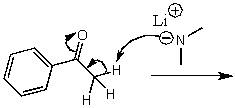
A)
B)
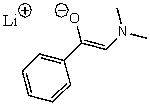
C)
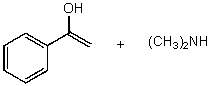
D)
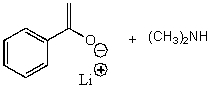
E) None of the above

A)
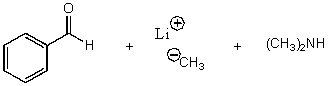
B)

C)

D)

E) None of the above

4
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 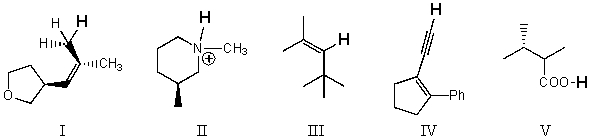
A) I > II > III > IV > V
B) V > IV > II > III > I
C) V > II > IV > III > I
D) II > V > IV > III > I
E) V > IV > III > II > I

A) I > II > III > IV > V
B) V > IV > II > III > I
C) V > II > IV > III > I
D) II > V > IV > III > I
E) V > IV > III > II > I

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
5
What is/are the product(s)of the following acid-base mechanism? 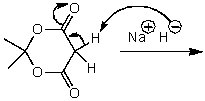
A)
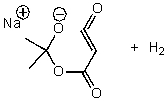
B)
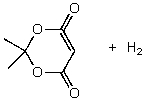
C)
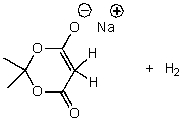
D)
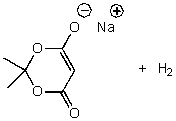
E) None of the above

A)

B)

C)

D)

E) None of the above

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
6
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 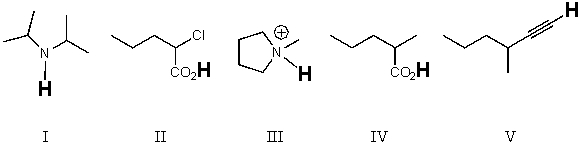
A) II > IV > V > III > I
B) IV > II > III > V > I
C) V > III > IV > II > I
D) II > IV > III > V > I
E) IV > II > I > V > III

A) II > IV > V > III > I
B) IV > II > III > V > I
C) V > III > IV > II > I
D) II > IV > III > V > I
E) IV > II > I > V > III

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
7
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 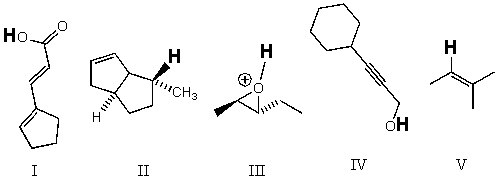
A) III > IV > I > V > II
B) II > III > V > IV > I
C) II > V > III > IV > I
D) V > III > II > IV > I
E) III > I > IV > V > II

A) III > IV > I > V > II
B) II > III > V > IV > I
C) II > V > III > IV > I
D) V > III > II > IV > I
E) III > I > IV > V > II

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
8
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 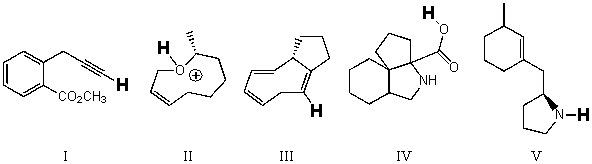
A) I > IV > II > V > III
B) II > IV > I > V > III
C) II > IV > V > I > III
D) IV > I > II > V > III
E) IV > II > I > V > III

A) I > IV > II > V > III
B) II > IV > I > V > III
C) II > IV > V > I > III
D) IV > I > II > V > III
E) IV > II > I > V > III

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
9
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 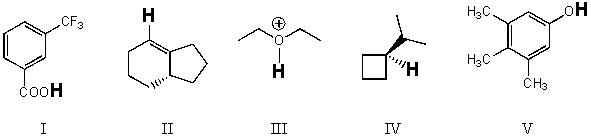
A) I > II > III > IV > V
B) III > V > II > I > IV
C) V > II > IV > III > I
D) III > I > V > II > IV
E) V > III > I > II > IV

A) I > II > III > IV > V
B) III > V > II > I > IV
C) V > II > IV > III > I
D) III > I > V > II > IV
E) V > III > I > II > IV

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
10
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 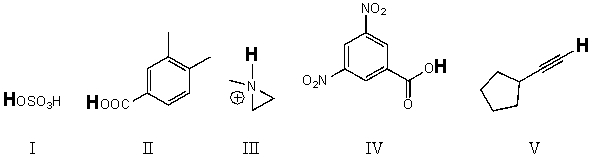
A) IV > II > III > V > I
B) II > IV > I > V > III
C) IV > I > III > II > V
D) I > IV > II > V > III
E) I > IV > II > III > V

A) IV > II > III > V > I
B) II > IV > I > V > III
C) IV > I > III > II > V
D) I > IV > II > V > III
E) I > IV > II > III > V

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
11
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 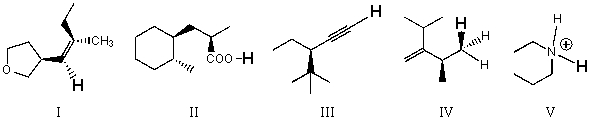
A) I > II > III > IV > V
B) II > V > III > I > IV
C) II > III > V > I > IV
D) III > I > V > II > IV
E) V > II > III > I > IV

A) I > II > III > IV > V
B) II > V > III > I > IV
C) II > III > V > I > IV
D) III > I > V > II > IV
E) V > II > III > I > IV

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
12
What is/are the product(s)of the following acid-base mechanism? 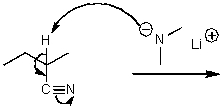
A)
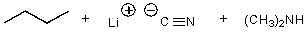
B)
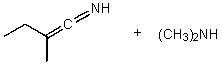
C)
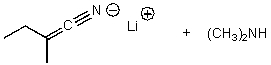
D)
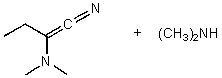
E) None of the above

A)
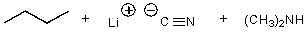
B)
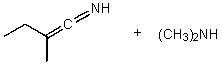
C)
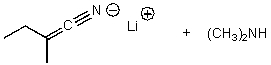
D)
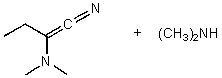
E) None of the above

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
13
What is/are the product(s)of the following acid-base mechanism? 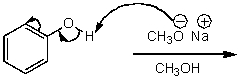
A)
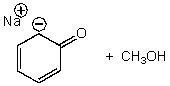
B)
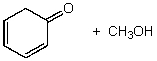
C)
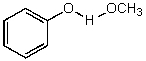
D)
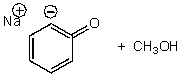
E) None of the above

A)

B)

C)

D)

E) None of the above

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
14
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 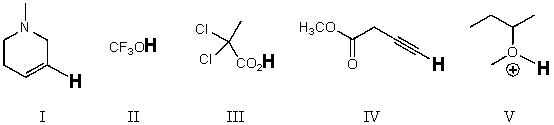
A) V > III > II > IV > I
B) II > V > III > I > IV
C) II > III > V > IV > I
D) III > I > V > II > IV
E) V > II > III > I > IV

A) V > III > II > IV > I
B) II > V > III > I > IV
C) II > III > V > IV > I
D) III > I > V > II > IV
E) V > II > III > I > IV

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
15
What is/are the product(s)of the following acid-base mechanism? 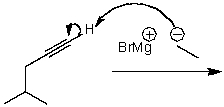
A)
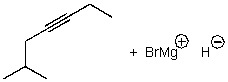
B)
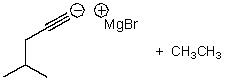
C)
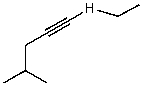
D)
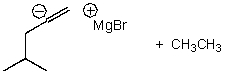
E) None of the above

A)

B)
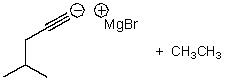
C)

D)
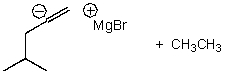
E) None of the above

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
16
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 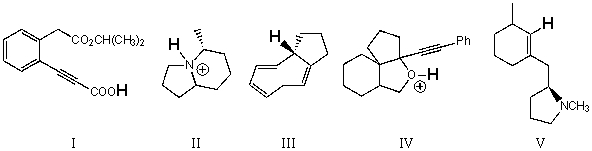
A) I > IV > II > V > III
B) II > I > IV > V > III
C) V > III > II > I > IV
D) IV > I > II > V > III
E) IV > II > I > V > III

A) I > IV > II > V > III
B) II > I > IV > V > III
C) V > III > II > I > IV
D) IV > I > II > V > III
E) IV > II > I > V > III

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
17
What is/are the product(s)of the following acid-base mechanism? 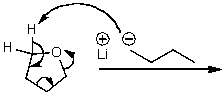
A)
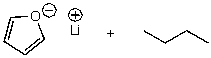
B)
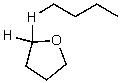
C)
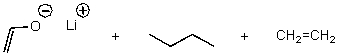
D)
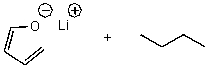
E) None of the above

A)
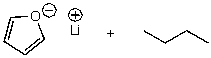
B)

C)

D)
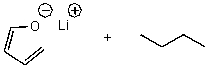
E) None of the above

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
18
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 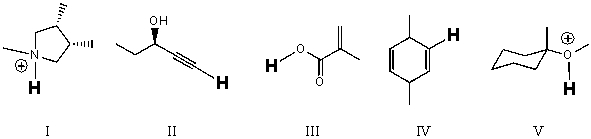
A) I > II > III > IV > V
B) III > V > II > I > IV
C) V > II > IV > III > I
D) III > I > V > II > IV
E) V > III > I > II > IV

A) I > II > III > IV > V
B) III > V > II > I > IV
C) V > II > IV > III > I
D) III > I > V > II > IV
E) V > III > I > II > IV

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
19
What is/are the product(s)of the following acid-base mechanism? 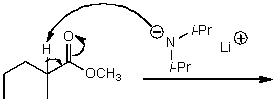
A)
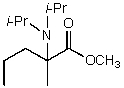
B)
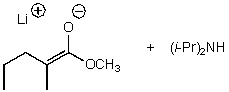
C)
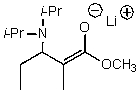
D)
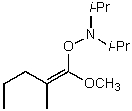
E) None of the above

A)

B)

C)

D)

E) None of the above

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
20
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 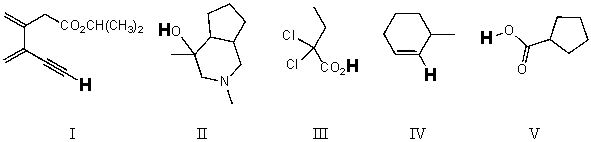
A) V > III > II > IV > I
B) II > V > III > I > IV
C) V > III > II > I > IV
D) III > I > V > II > IV
E) III > V > II > I > IV

A) V > III > II > IV > I
B) II > V > III > I > IV
C) V > III > II > I > IV
D) III > I > V > II > IV
E) III > V > II > I > IV

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
21
What reactants would give the following products? 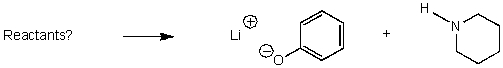
A)
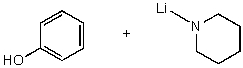
B)
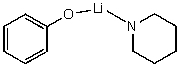
C)
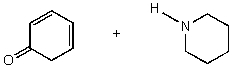
D)
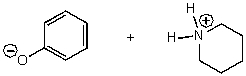
E) No reaction

A)
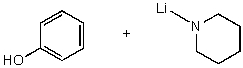
B)
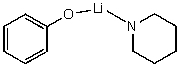
C)
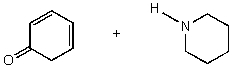
D)
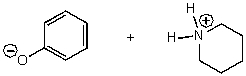
E) No reaction

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
22
What does the reaction between the following two species produce? 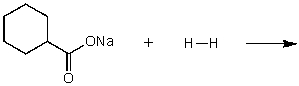
A)
B)
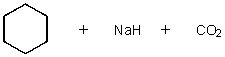
C)
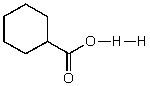
D)
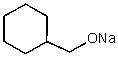
E) No reaction
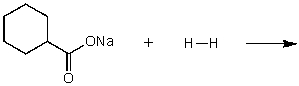
A)

B)
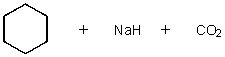
C)

D)

E) No reaction

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
23
The process of bond-breaking where each fragment takes away one of the electrons from the bond is called ____________.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
24
What does the reaction between the following two species produce? 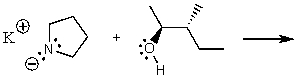
A)
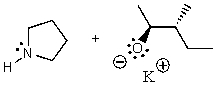
B)
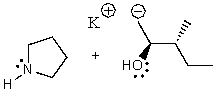
C)
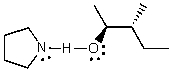
D)
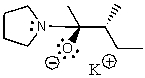
E) No reaction

A)

B)

C)

D)

E) No reaction

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
25
The four basic types of reactions are: ________________.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
26
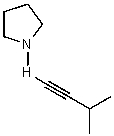
What does the reaction between the following two species produce? 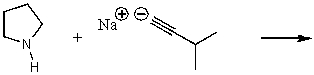
A)
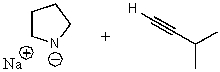
B)
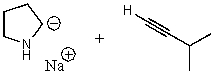
C)
D)
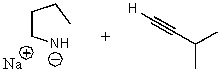
E) No reaction

A)
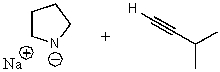
B)
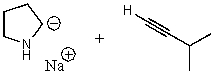
C)

D)
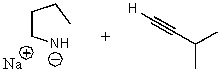
E) No reaction

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
27
Which base would effectively deprotonate benzoic acid (PhCOOH)?
A) NaCl
B) H2O
C) NaO2CCH3
D) t-BuOK
E) They would all effectively deprotonate benzoic acid.
A) NaCl
B) H2O
C) NaO2CCH3
D) t-BuOK
E) They would all effectively deprotonate benzoic acid.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
28
What is the major product for the following reaction? 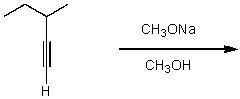
A)
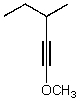
B)
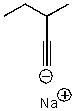
C)
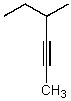
D)
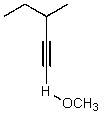
E) No reaction
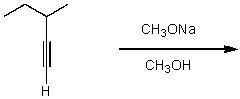
A)

B)

C)

D)

E) No reaction

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
29
What reactants would give the following products? 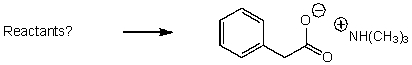
A)
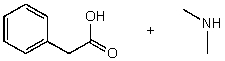
B)
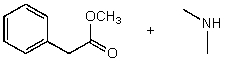
C)
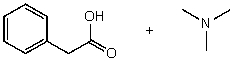
D)
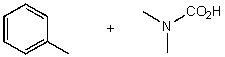
E) None of the above

A)

B)

C)

D)
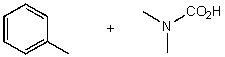
E) None of the above

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
30
Which base would not effectively deprotonate benzoic acid (PhCOOH)?
A) i-PrMgBr
B) NaOH
C) NaH
D) (i-Pr)2NLi
E) They would all effectively deprotonate benzoic acid.
A) i-PrMgBr
B) NaOH
C) NaH
D) (i-Pr)2NLi
E) They would all effectively deprotonate benzoic acid.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
31
Which base would not effectively deprotonate phenol (PhOH)?
A) i-PrMgBr
B) H2O
C) NaH
D) (i-Pr)2NLi
E) t-BuOK
A) i-PrMgBr
B) H2O
C) NaH
D) (i-Pr)2NLi
E) t-BuOK

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the reaction conditions could afford the following transfromation? 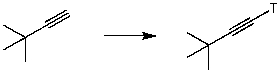
A)
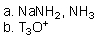
B)
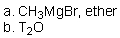
C)
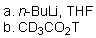
D)
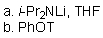
E) All of the above would work.

A)
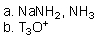
B)
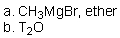
C)
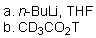
D)
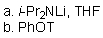
E) All of the above would work.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
33
Which base would not effectively deprotonate acetylene?
A) i-PrMgBr
B) KH
C) (CH3O)2CH2Li
D) (i-Pr)2NLi
E) t-BuOK
A) i-PrMgBr
B) KH
C) (CH3O)2CH2Li
D) (i-Pr)2NLi
E) t-BuOK

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
34
Which combination of reagents is the least effective in generating sodium isopropoxide, (CH3)2CH2ONa?
A) (CH3)2CH2OH + CH3Na
B) (CH3)2CH2OH + NaNH2
C) (CH3)2CH2OH + NaH
D) (CH3)2CH2OH + H2C=CHNa
E) (CH3)2CH2OH + CH3CH2ONa
A) (CH3)2CH2OH + CH3Na
B) (CH3)2CH2OH + NaNH2
C) (CH3)2CH2OH + NaH
D) (CH3)2CH2OH + H2C=CHNa
E) (CH3)2CH2OH + CH3CH2ONa

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
35
What is the major product for the following reaction? 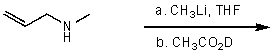
A)
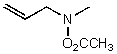
B)
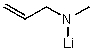
C)
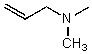
D)
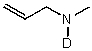
E) No reaction
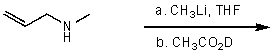
A)

B)

C)

D)

E) No reaction

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
36
What is the major product for the following reaction? 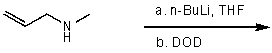
A)
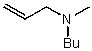
B)
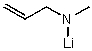
C)
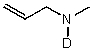
D) LiD2O
E) No Reaction
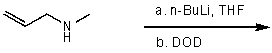
A)

B)

C)

D) LiD2O
E) No Reaction

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
37
Which base would not effectively deprotonate acetylene?
A) LiOCH3
B) CH3Li
C) CH3OCH2MgBr
D) KH
E) (CH3)2NLi
A) LiOCH3
B) CH3Li
C) CH3OCH2MgBr
D) KH
E) (CH3)2NLi

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
38
Reagents that seek to react with a proton or some other electron-deficient center are called ____________.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
39
Addition reactions are characteristic of compounds with ______________.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the reaction conditions would not afford the following transfromation? 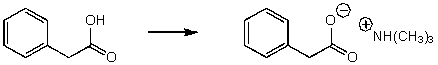
A) (CH3)2NH
B) (CH3)3N
C) (CH3)2NLi
D) More than one
E) None of the above

A) (CH3)2NH
B) (CH3)3N
C) (CH3)2NLi
D) More than one
E) None of the above

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
41
Isotope labeling is an important tool in the study of reaction mechanisms.How will you selectively deuterate the specified hydrogen atom,indicated by an arrow,in the following compound? Use equations to clarify your answer and briefly explain your rationale. 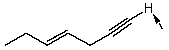


Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
42
Briefly,but clearly,explain why the -OH hydrogen in acetic acid (CH3CO2H)is more acidic than in ethanol (C2H5OH).

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
43
Write an equation to show the reaction between ethanol,C2H5OH and methyllithium,CH3Li.Draw all non-bonding electrons and show electron flow with curved arrows.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
44
Define a protic solvent.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
45
Bond polarization that takes place through space and through the bonds of the molecule is called the _____________.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
46
You are planning to carry out a reaction between propyne,CH3C≡CH and sodium amide,NaNH2.You also need to choose an appropriate solvent for carrying out the reaction.Would ethanol be suitable for this purpose? Explain your rationale clearly.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
47
What are the two fundamental types of energy?

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck


