Exam 3: An Introduction to Organic Reactions: Acids and Bases
Exam 1: Carbon Compounds and Chemical Bonds134 Questions
Exam 2: Representative Carbon Compounds: Functional Groups, Intermolecular Forces, and Infrared Ir Spectroscopy114 Questions
Exam 3: An Introduction to Organic Reactions: Acids and Bases47 Questions
Exam 4: Alkanes: Nomenclature, Conformational Analysis, and an Introduction to Synthesis125 Questions
Exam 5: Stereochemistry: Chiral Molecules150 Questions
Exam 6: Ionic Reactions - Nucleophilic Substitution and Elimination Reactions of Alkyl Halides146 Questions
Exam 7: Alkenes and Alkynes I: Properties and Synthesis, Elimination Reactions of Alkyl Halides99 Questions
Exam 8: Alkenes and Alkynes Ii: Addition Reactions140 Questions
Exam 9: Nuclear Magnetic Resonance and Mass Spectrometry: Tools for Structure Determination94 Questions
Exam 10: Radical Reactions114 Questions
Exam 11: Alcohols and Ethers172 Questions
Exam 12: Alcohols From Carbonyl Compounds Oxidation-Reduction and Organometallic Compounds147 Questions
Exam 13: Conjugated Unsaturated Systems166 Questions
Exam 14: Aromatic Compounds151 Questions
Exam 15: Reactions of Aromatic Compounds173 Questions
Exam 16: Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group165 Questions
Exam 17: Aldehydes and Ketones Ii Aldol Reactions131 Questions
Exam 18: Carboxylic Acids and Their Derivatives Nucleophilic Addition - Elimination at the Acyl Carbon124 Questions
Exam 19: Synthesis and Reactions of Beta-Dicarbonyl Compounds: More Chemistry of Enolate Ions131 Questions
Exam 20: Amines148 Questions
Exam 21: Phenols and Aryl Halides: Nucleophilic Aromatic Substitution87 Questions
Exam 22: Carbohydrates104 Questions
Exam 23: Lipids99 Questions
Exam 24: Amino Acids and Proteins94 Questions
Exam 25: Nucleic Acids and Protein Synthesis89 Questions
Select questions type
The four basic types of reactions are: ________________.
Free
(Short Answer)
4.7/5  (32)
(32)
Correct Answer:
substitution,addition,elimination,rearrangement
What does the reaction between the following two species produce? 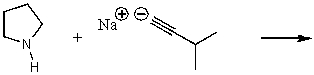
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
E
What is/are the product(s)of the following acid-base mechanism? 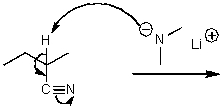
Free
(Multiple Choice)
4.8/5  (41)
(41)
Correct Answer:
E
Which base would not effectively deprotonate benzoic acid (PhCOOH)?
(Multiple Choice)
4.8/5  (34)
(34)
Addition reactions are characteristic of compounds with ______________.
(Short Answer)
4.8/5  (35)
(35)
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 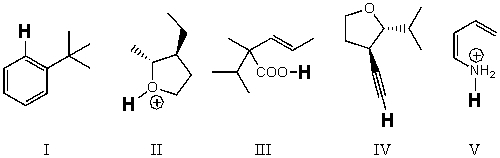
(Multiple Choice)
4.7/5  (34)
(34)
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 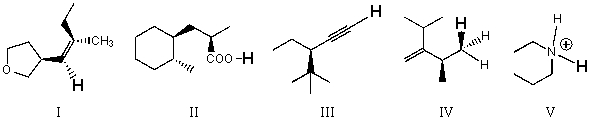
(Multiple Choice)
4.9/5  (37)
(37)
What is/are the product(s)of the following acid-base mechanism? 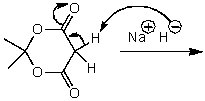
(Multiple Choice)
4.8/5  (35)
(35)
What is/are the product(s)of the following acid-base mechanism? 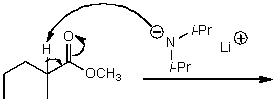
(Multiple Choice)
4.9/5  (40)
(40)
Reagents that seek to react with a proton or some other electron-deficient center are called ____________.
(Short Answer)
4.8/5  (45)
(45)
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 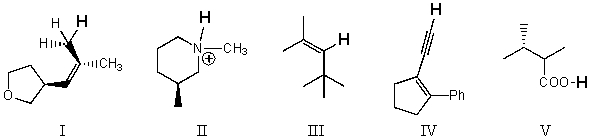
(Multiple Choice)
4.8/5  (37)
(37)
The process of bond-breaking where each fragment takes away one of the electrons from the bond is called ____________.
(Short Answer)
4.9/5  (44)
(44)
Write an equation to show the reaction between ethanol,C2H5OH and methyllithium,CH3Li.Draw all non-bonding electrons and show electron flow with curved arrows.
(Essay)
4.8/5  (34)
(34)
What is/are the product(s)of the following acid-base mechanism? 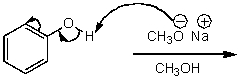
(Multiple Choice)
5.0/5  (41)
(41)
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 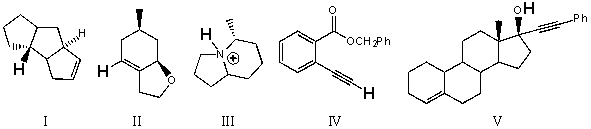
(Multiple Choice)
4.8/5  (35)
(35)
Isotope labeling is an important tool in the study of reaction mechanisms.How will you selectively deuterate the specified hydrogen atom,indicated by an arrow,in the following compound? Use equations to clarify your answer and briefly explain your rationale. 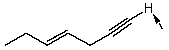
(Essay)
4.7/5  (35)
(35)
What is/are the product(s)of the following acid-base mechanism? 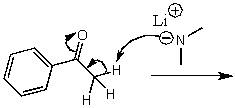
(Multiple Choice)
4.9/5  (40)
(40)
Showing 1 - 20 of 47
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)