Deck 6: Ionic Reactions - Nucleophilic Substitution and Elimination Reactions of Alkyl Halides
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/146
Play
Full screen (f)
Deck 6: Ionic Reactions - Nucleophilic Substitution and Elimination Reactions of Alkyl Halides
1
Select the potential energy diagram that represents an exothermic (exergonic)reaction. 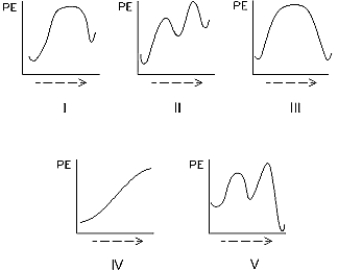
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V
I
2
Consider the reaction of 2-chloro-2-methylpentane with sodium iodide. 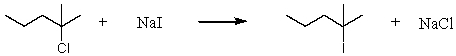 Assuming no other changes,how would it affect the rate if one simultaneously doubled the concentration of 2-chloro-2-methylpentane and sodium iodide?
Assuming no other changes,how would it affect the rate if one simultaneously doubled the concentration of 2-chloro-2-methylpentane and sodium iodide?
A) No effect
B) It would double the rate.
C) It would triple the rate.
D) It would quadruple the rate.
E) It would increase the rate five times.
 Assuming no other changes,how would it affect the rate if one simultaneously doubled the concentration of 2-chloro-2-methylpentane and sodium iodide?
Assuming no other changes,how would it affect the rate if one simultaneously doubled the concentration of 2-chloro-2-methylpentane and sodium iodide?A) No effect
B) It would double the rate.
C) It would triple the rate.
D) It would quadruple the rate.
E) It would increase the rate five times.
It would double the rate.
3
The reaction, 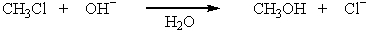 has the following thermodynamic values at 27ºC: H = -75.3 kJ mol-1; S = 54.4 J K-1 mol-1.What is the value of G for this reaction?
has the following thermodynamic values at 27ºC: H = -75.3 kJ mol-1; S = 54.4 J K-1 mol-1.What is the value of G for this reaction?
A) -73.8 kJ mol-1
B) -76.8 kJ mol-1
C) -59.0 kJ mol-1
D) +91.6 kJ mol-1
E) -91.6 kJ mol-1
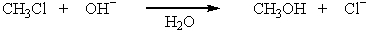 has the following thermodynamic values at 27ºC: H = -75.3 kJ mol-1; S = 54.4 J K-1 mol-1.What is the value of G for this reaction?
has the following thermodynamic values at 27ºC: H = -75.3 kJ mol-1; S = 54.4 J K-1 mol-1.What is the value of G for this reaction?A) -73.8 kJ mol-1
B) -76.8 kJ mol-1
C) -59.0 kJ mol-1
D) +91.6 kJ mol-1
E) -91.6 kJ mol-1
-91.6 kJ mol-1
4
Increasing the temperature of a chemical reaction usually increases greatly the rate of the reaction.The primary reason for this is that increasing the temperature:
A) Decreases the energy of activation.
B) Increases the total number of collisions between reactants.
C) Decreases the rate of the reverse reaction.
D) Favors endothermic processes.
E) None of the above properly explains the observed increase in reaction rates with increase in temperature.
A) Decreases the energy of activation.
B) Increases the total number of collisions between reactants.
C) Decreases the rate of the reverse reaction.
D) Favors endothermic processes.
E) None of the above properly explains the observed increase in reaction rates with increase in temperature.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
5
The rate equation for a nucleophilic substitution reaction of a secondary alkyl chloride (R-Cl)with I-ion would be:
A) Rate = k [RCl]
B) Rate = k [I-]
C) Rate = k [RCl][I-]
D) Rate = k [RCl]2[I - ]
E) Rate = k [RCl][I - ]2
A) Rate = k [RCl]
B) Rate = k [I-]
C) Rate = k [RCl][I-]
D) Rate = k [RCl]2[I - ]
E) Rate = k [RCl][I - ]2

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
6
An increase in the temperature at which a reaction is carried out increases:
A) the collision frequency.
B) the fraction of molecules with proper orientation.
C) the fraction of molecules with energy greater than Eact.
D) More than one of the above
E) None of the above
A) the collision frequency.
B) the fraction of molecules with proper orientation.
C) the fraction of molecules with energy greater than Eact.
D) More than one of the above
E) None of the above

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
7
Increasing the temperature of a chemical reaction usually increases greatly the rate of the reaction.The most important reason for this is that increasing the temperature:
A) Increases the collision frequency.
B) Decreases the probability factor.
C) Increases the fraction of collisions with energy greater than Eact.
D) Decreases the energy of activation.
E) Makes the reaction more exothermic.
A) Increases the collision frequency.
B) Decreases the probability factor.
C) Increases the fraction of collisions with energy greater than Eact.
D) Decreases the energy of activation.
E) Makes the reaction more exothermic.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
8
Consider the SN2 reaction of butyl bromide with OH- ion. CH3CH2CH2CH2Br + OH- CH3CH2CH2CH2OH + Br-
Assuming no other changes,what effect on the rate would result from simultaneously doubling the concentrations of both butyl bromide and OH- ion?
A) No effect.
B) It would double the rate.
C) It would triple the rate.
D) It would increase the rate four times.
E) It would increase the rate six times.
Assuming no other changes,what effect on the rate would result from simultaneously doubling the concentrations of both butyl bromide and OH- ion?
A) No effect.
B) It would double the rate.
C) It would triple the rate.
D) It would increase the rate four times.
E) It would increase the rate six times.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
9
Which will be true for any actual or potential nucleophilic substitution reaction?
A) " H is positive."
B) " H is negative."
C) " G‡ is positive."
D) " G is positive."
E) " G is negative."
A) " H is positive."
B) " H is negative."
C) " G‡ is positive."
D) " G is positive."
E) " G is negative."

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
10
For the typical SN2 reaction Y- + RX RY + X-
It can be predicted that S‡ will be:
A) positive.
B) zero.
C) negative.
D) either positive or negative.
E) unpredictable as to algebraic sign.
It can be predicted that S‡ will be:
A) positive.
B) zero.
C) negative.
D) either positive or negative.
E) unpredictable as to algebraic sign.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
11
What product(s)would you expect to obtain from the following SN2 reaction? 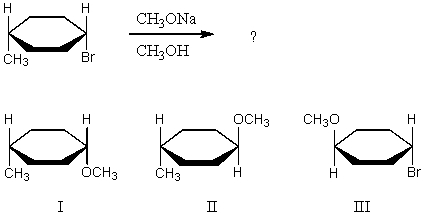
A) I
B) II
C) An equimolar mixture of I and II.
D) III
E) None of these

A) I
B) II
C) An equimolar mixture of I and II.
D) III
E) None of these

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
12
Consider the SN2 reaction of 2-iodopentane with CH3CO2- ion. 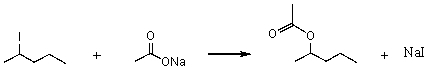 Assuming no other changes,what effect on the rate would result from simultaneously doubling the concentrations of both 2-iodopentane and CH3CO2- ion?
Assuming no other changes,what effect on the rate would result from simultaneously doubling the concentrations of both 2-iodopentane and CH3CO2- ion?
A) No effect.
B) It would double the rate.
C) It would triple the rate.
D) It would increase the rate four times.
E) It would increase the rate six times.
 Assuming no other changes,what effect on the rate would result from simultaneously doubling the concentrations of both 2-iodopentane and CH3CO2- ion?
Assuming no other changes,what effect on the rate would result from simultaneously doubling the concentrations of both 2-iodopentane and CH3CO2- ion?A) No effect.
B) It would double the rate.
C) It would triple the rate.
D) It would increase the rate four times.
E) It would increase the rate six times.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
13
The rate equation for a nucleophilic substitution reaction of a tertiary alkyl bromide (R-Br)with I - ion would be:
A) Rate = k [RBr]
B) Rate = k [I - ]
C) Rate = k [RBr][I - ]
D) Rate = k [RBr]2[I - ]
E) Rate = k [RBr][I - ]2
A) Rate = k [RBr]
B) Rate = k [I - ]
C) Rate = k [RBr][I - ]
D) Rate = k [RBr]2[I - ]
E) Rate = k [RBr][I - ]2

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
14
Select the potential energy diagram that represents a two-step endothermic (endergonic)reaction. 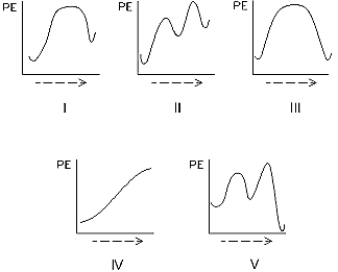
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
15
A true statement about the transition state(s)of an SN2 reaction is:
A) the two transition states are of unequal energy.
B) the transition states precede and follow an unstable reaction intermediate.
C) the single transition state represents the point of maximum free energy of the reaction.
D) existence of this transition state implies an exothermic reaction.
E) the transition state will always have a net charge of -1.
A) the two transition states are of unequal energy.
B) the transition states precede and follow an unstable reaction intermediate.
C) the single transition state represents the point of maximum free energy of the reaction.
D) existence of this transition state implies an exothermic reaction.
E) the transition state will always have a net charge of -1.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
16
The hybridization state of the charged carbon in a carbocation is
A) sp4
B) sp3
C) sp2
D) sp
E) s
A) sp4
B) sp3
C) sp2
D) sp
E) s

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
17
The major product of the following reaction would be: 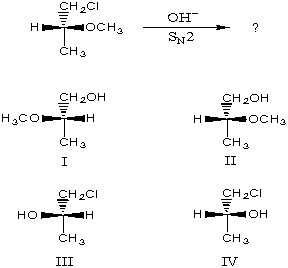
A) I
B) II
C) III
D) IV
E) An equimolar mixture of I and II.

A) I
B) II
C) III
D) IV
E) An equimolar mixture of I and II.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
18
Select the rate law for the following reaction,e.g. , CH3CH2CH2CHBrCH3 + OH - CH3CH2CH2CHOHCH3 + X -
( RBr )
A) Rate = k [RBr]
B) Rate = k [RBr] [OH - ]
C) Rate = k [RBr]2 [OH - ]
D) Rate = k [RBr] [OH - ]2
E) Rate = k [RBr]2 [OH - ]2
( RBr )
A) Rate = k [RBr]
B) Rate = k [RBr] [OH - ]
C) Rate = k [RBr]2 [OH - ]
D) Rate = k [RBr] [OH - ]2
E) Rate = k [RBr]2 [OH - ]2

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
19
Consider the SN2 reaction of 1-chloro-5-methylhexane with CN-ion. 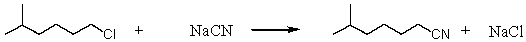 Assuming no other changes,what effect on the rate would result from simultaneously doubling the concentrations of both 1-chloro-5-methylhexane and CN- ion?
Assuming no other changes,what effect on the rate would result from simultaneously doubling the concentrations of both 1-chloro-5-methylhexane and CN- ion?
A) No effect.
B) It would double the rate.
C) It would triple the rate.
D) It would increase the rate four times.
E) It would increase the rate six times.
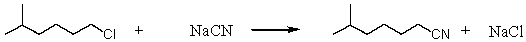 Assuming no other changes,what effect on the rate would result from simultaneously doubling the concentrations of both 1-chloro-5-methylhexane and CN- ion?
Assuming no other changes,what effect on the rate would result from simultaneously doubling the concentrations of both 1-chloro-5-methylhexane and CN- ion?A) No effect.
B) It would double the rate.
C) It would triple the rate.
D) It would increase the rate four times.
E) It would increase the rate six times.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
20
The difference in the bond energies of reactants and the transition state of a reaction is designated by the notation:
A) " H "
B) " H‡"
C) " G "
D) " G‡"
E) " S‡"
A) " H "
B) " H‡"
C) " G "
D) " G‡"
E) " S‡"

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
21
Which alkyl halide would you expect to undergo an SN2 reaction most slowly?
A) 1-bromohexane
B) 1-bromo-2-methylpentane
C) 1-bromo-3-methylpentane
D) 1-bromo-4-methylpentane
E) 1-bromo-2,2-dimethylbutane
A) 1-bromohexane
B) 1-bromo-2-methylpentane
C) 1-bromo-3-methylpentane
D) 1-bromo-4-methylpentane
E) 1-bromo-2,2-dimethylbutane

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
22
The p orbital of a methyl cation,CH3+,contains how many electrons?
A) 1
B) 2
C) 3
D) 4
E) 0
A) 1
B) 2
C) 3
D) 4
E) 0

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
23
By analyzing the starting material and the product(s),the following reaction is an example of what type of mechanism? 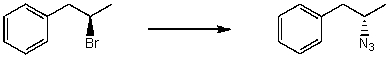
A) SN1
B) SN2
C) E1
D) E2
E) More than one of the above

A) SN1
B) SN2
C) E1
D) E2
E) More than one of the above

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
24
By analyzing the starting material and the product(s),the following reaction is an example of what type of mechanism? 
A) SN1
B) SN2
C) E1
D) E2
E) None of the above

A) SN1
B) SN2
C) E1
D) E2
E) None of the above

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
25
Which is the strongest nucleophile?
A) OH-
B) CH3CH2O-
C)
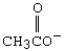
D) CH3CH2OH
E) H2O
A) OH-
B) CH3CH2O-
C)
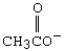
D) CH3CH2OH
E) H2O

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
26
Ambident nucleophiles are ones which can react with a substrate at either of two nucleophilic sites.Which of the following is not an ambident nucleophile? 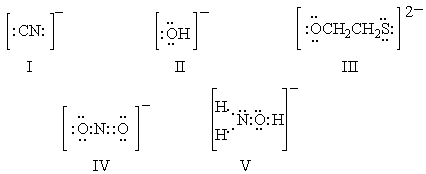
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
27
By analyzing the starting material and the product(s),the following reaction is an example of what type of mechanism? 
A) SN1
B) SN2
C) E1
D) E2
E) None of the above

A) SN1
B) SN2
C) E1
D) E2
E) None of the above

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
28
An increase in the kinetic energy of reacting molecules results in:
A) a decrease in reaction rate.
B) an increase in the probability factor.
C) a decrease in the probability factor.
D) an increase in the reaction rate.
E) no changes.
A) a decrease in reaction rate.
B) an increase in the probability factor.
C) a decrease in the probability factor.
D) an increase in the reaction rate.
E) no changes.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
29
Which alkyl chloride,though primary,is essentially unreactive in SN2 reactions?
A)

B)
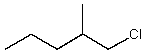
C)
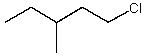
D)
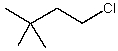
E)
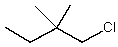
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
30
Which is a true statement concerning the transition state of the rate-determining step of an SN1 reaction?
A) Structurally,it closely resembles the carbocation intermediate.
B) Both covalent bond-breaking and bond-making are occurring.
C) Formation of the transition state is an exothermic reaction.
D) Necessarily,the transition state has zero charge overall.
E) More than one of the above.
A) Structurally,it closely resembles the carbocation intermediate.
B) Both covalent bond-breaking and bond-making are occurring.
C) Formation of the transition state is an exothermic reaction.
D) Necessarily,the transition state has zero charge overall.
E) More than one of the above.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
31
Which SN2 reaction would you expect to take place most rapidly? Assume that the concentrations of the reactants and the temperature are the same in each instance:
A)

B)
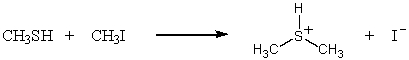
C)

D)
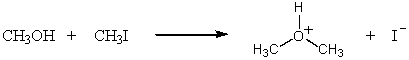
E)

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
32
You want to synthesize 3-methyl-2-pentene from 2-chloro-3-methylpentane.Which reagent would you use?
A) HCl,heat
B) NH3(aq),25oC
C) CH3CO2Na,CH3CO2H,heat
D) CH3CH2ONa,CH3CH2OH,heat
E) CH3CH2OH,heat
A) HCl,heat
B) NH3(aq),25oC
C) CH3CO2Na,CH3CO2H,heat
D) CH3CH2ONa,CH3CH2OH,heat
E) CH3CH2OH,heat

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following reactions proceeds with inversion of configuration at the carbon bearing the leaving group?
A) SN2
B) SN1
C) E2
D) E1
E) All of these
A) SN2
B) SN1
C) E2
D) E1
E) All of these

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
34
The Hammond-Leffler postulate,when applied to nucleophilic substitutions and elimination reactions,states that:
A) a negatively-charged nucleophile is stronger than its conjugate acid.
B) polar aprotic solvents strongly accelerate the rate of SN2 processes.
C) bimolecular nucleophilic substitutions are 2nd order kinetically.
D) the transition state for an endergonic reaction step (one accompanied by an increase in free energy)resembles the product of that step.
E) elimination reactions will always compete with nucleophilic substitution reactions.
A) a negatively-charged nucleophile is stronger than its conjugate acid.
B) polar aprotic solvents strongly accelerate the rate of SN2 processes.
C) bimolecular nucleophilic substitutions are 2nd order kinetically.
D) the transition state for an endergonic reaction step (one accompanied by an increase in free energy)resembles the product of that step.
E) elimination reactions will always compete with nucleophilic substitution reactions.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
35
Consider the substitution reaction that takes place when (R)-3-bromo-3-methylhexane is treated with sodium methoxide.Which of the following would be true?
A) An SN2 reaction would take place with inversion of configuration at the stereogenic center.
B) An SN1 reaction would take place with retention of configuration at the stereogenic center.
C) An SN1 reaction would take place with racemization of configuration at the stereogenic center.
D) An SN1 reaction would take place,accompanied by an E1 reaction,affording a complex mixture of products.
E) An E2 reaction would take place,during which the stereogenic center is lost.
A) An SN2 reaction would take place with inversion of configuration at the stereogenic center.
B) An SN1 reaction would take place with retention of configuration at the stereogenic center.
C) An SN1 reaction would take place with racemization of configuration at the stereogenic center.
D) An SN1 reaction would take place,accompanied by an E1 reaction,affording a complex mixture of products.
E) An E2 reaction would take place,during which the stereogenic center is lost.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
36
By analyzing the starting material and the product(s),the following reaction is possibly an example of what type of mechanism? 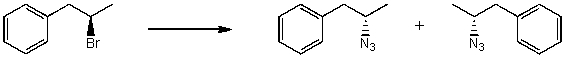
A) SN1
B) SN2
C) E1
D) E2
E) More than one of the above

A) SN1
B) SN2
C) E1
D) E2
E) More than one of the above

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
37
Consider the substitution reaction that takes place when (R)-3-bromo-3-methylhexane is treated with methanol.Which of the following would be true?
A) The reaction would take place only with inversion of configuration at the stereogenic center.
B) The reaction would take place only with retention of configuration at the stereogenic center.
C) The reaction would take place with racemization.
D) No reaction would take place.
E) The alkyl halide does not possess a stereogenic center.
A) The reaction would take place only with inversion of configuration at the stereogenic center.
B) The reaction would take place only with retention of configuration at the stereogenic center.
C) The reaction would take place with racemization.
D) No reaction would take place.
E) The alkyl halide does not possess a stereogenic center.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
38
Consider the substitution reaction that takes place when (R)-3-iodo-3-methylheptane is treated with sodium acetate (CH3CO2Na).Which of the following would be true?
A) An SN2 reaction would take place with inversion of configuration at the stereogenic center.
B) An SN1 reaction would take place with retention of configuration at the stereogenic center.
C) An SN1 reaction would take place with racemization of configuration at the stereogenic center.
D) An SN1 reaction would take place,accompanied by an E1 reaction,affording a complex mixture of products.
E) An E2 reaction would take place,during which the stereogenic center is lost.
A) An SN2 reaction would take place with inversion of configuration at the stereogenic center.
B) An SN1 reaction would take place with retention of configuration at the stereogenic center.
C) An SN1 reaction would take place with racemization of configuration at the stereogenic center.
D) An SN1 reaction would take place,accompanied by an E1 reaction,affording a complex mixture of products.
E) An E2 reaction would take place,during which the stereogenic center is lost.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
39
The p orbital of the charged carbon in the isopropyl cation, (CH3)2CH+,contains how many electrons?
A) 1
B) 2
C) 3
D) 4
E) 0
A) 1
B) 2
C) 3
D) 4
E) 0

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
40
Which alkyl halide would be most reactive in an SN1 reaction? 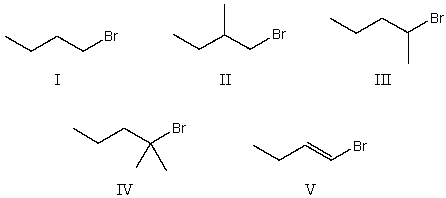
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following is the poorest leaving group?
A) H -
B) CH3O -
C) H2O
D) OH -
E) NH2 -
A) H -
B) CH3O -
C) H2O
D) OH -
E) NH2 -

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
42
Which is the weakest nucleophile in polar protic solvents?
A) I-
B) Br-
C) Cl-
D) F-
E) All of the above are equally strong nucleophiles,regardless of the type of solvent used.
A) I-
B) Br-
C) Cl-
D) F-
E) All of the above are equally strong nucleophiles,regardless of the type of solvent used.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
43
Which SN2 reaction would take place most rapidly?
A)
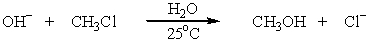
B)
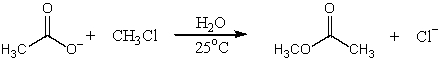
C)
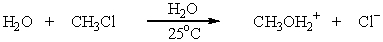
D)
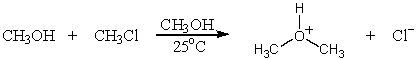
E)
A)
B)
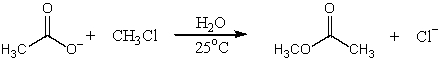
C)
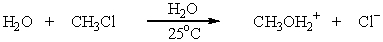
D)
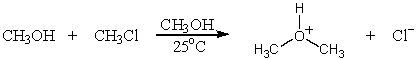
E)
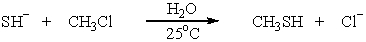

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
44
Identify the leaving group in the following reaction: 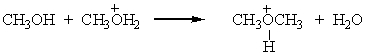
A) CH3OH
B) CH3OH2+
C) CH3OCH3
D) H2O
E) None of these

A) CH3OH
B) CH3OH2+
C) CH3OCH3
D) H2O
E) None of these

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
45
Which alkyl halide would you expect to react most slowly when heated in aqueous solution?
A) (CH3)3C-F
B) (CH3)3C-Cl
C) (CH3)3C-Br
D) (CH3)3C-I
E) They would all react at the same rate.
A) (CH3)3C-F
B) (CH3)3C-Cl
C) (CH3)3C-Br
D) (CH3)3C-I
E) They would all react at the same rate.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
46
Which of these species,acting in a protic solvent,exhibits greater nucleophilic activity than expected on the basis of its basicity?
A) OH-
B) CH3O-
C) SH-
D) Cl-
E) H2O
A) OH-
B) CH3O-
C) SH-
D) Cl-
E) H2O

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following is not a good leaving group?
A) C2H5O -
B) Cl -
C) I -
D) CH3CO2 -
E) All of these are good leaving groups.
A) C2H5O -
B) Cl -
C) I -
D) CH3CO2 -
E) All of these are good leaving groups.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
48
Which SN2 reaction will occur most rapidly in a mixture of water and ethanol?
A) I- + CH3CH2-Br CH3CH2-I + Br-
B) I- + CH3CH2-Cl CH3CH2-I + Cl-
C) I- + CH3CH2-F CH3CH2-I + F-
D) Br- + CH3CH2-Cl CH3CH2-Br + Cl-
E) Br- + CH3CH2-F CH3CH2-Br + F-
A) I- + CH3CH2-Br CH3CH2-I + Br-
B) I- + CH3CH2-Cl CH3CH2-I + Cl-
C) I- + CH3CH2-F CH3CH2-I + F-
D) Br- + CH3CH2-Cl CH3CH2-Br + Cl-
E) Br- + CH3CH2-F CH3CH2-Br + F-

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
49
Which nucleophilic substitution reaction would be unlikely to occur?
A) HO - + CH3CH2-I CH3CH2-OH + I -
B) I - + CH3CH2-H CH3CH2-I + H -
C) CH3S - + CH3-Br CH3S-CH3 + Br -
D) All of the above would be unlikely to occur.
E) None of the above would be unlikely to occur.
A) HO - + CH3CH2-I CH3CH2-OH + I -
B) I - + CH3CH2-H CH3CH2-I + H -
C) CH3S - + CH3-Br CH3S-CH3 + Br -
D) All of the above would be unlikely to occur.
E) None of the above would be unlikely to occur.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
50
Which is the weakest nucleophile in polar aprotic solvents?
A) I-
B) Br-
C) Cl-
D) F-
E) All of the above are equally strong nucleophiles,regardless of the type of solvent used
A) I-
B) Br-
C) Cl-
D) F-
E) All of the above are equally strong nucleophiles,regardless of the type of solvent used

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
51
Which SN2 reaction will occur most rapidly in aqueous acetone solution? Assume concentrations and temperature are the same in each instance.
A) HO- + CH3-Cl CH3OH + Cl-
B) HO- + CH3CH2-Cl CH3CH2OH + Cl-
C) HO- + (CH3)2CH-Cl (CH3)2CHOH + Cl-
D) HO- + (CH3)3C-Cl (CH3)3COH + Cl-
E) HO- + (CH3)3CCH2-Cl (CH3)3CCH2OH + Cl-
A) HO- + CH3-Cl CH3OH + Cl-
B) HO- + CH3CH2-Cl CH3CH2OH + Cl-
C) HO- + (CH3)2CH-Cl (CH3)2CHOH + Cl-
D) HO- + (CH3)3C-Cl (CH3)3COH + Cl-
E) HO- + (CH3)3CCH2-Cl (CH3)3CCH2OH + Cl-

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
52
Which ion is the strongest nucleophile in an aprotic solvent such as dimethylsulfoxide?
A) I-
B) Br-
C) Cl-
D) F-
E) These are all equal.
A) I-
B) Br-
C) Cl-
D) F-
E) These are all equal.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following is a feasible substitution reaction?
A) CH3CH2Cl + NaBr CH3CH2Br + NaCl
B) CH3CH3 + NaCN CH3CH2CN + NaH
C) CH3CH2Cl + NaOH CH2=CH2 + H2O + NaCl
D) More than one of the above.
E) None of the above.
A) CH3CH2Cl + NaBr CH3CH2Br + NaCl
B) CH3CH3 + NaCN CH3CH2CN + NaH
C) CH3CH2Cl + NaOH CH2=CH2 + H2O + NaCl
D) More than one of the above.
E) None of the above.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
54
Which is the most reactive nucleophile in DMF (structure shown below)? 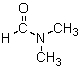
A) F-
B) Cl-
C) Br-
D) I-
E) They are all equally reactive.

A) F-
B) Cl-
C) Br-
D) I-
E) They are all equally reactive.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
55
Considering the relative solvation of reactants and the transition states of substitution reactions of these reactants,predict which general type of reaction would be most favored by the use of a polar solvent.
A) Y: + RX RY+ + X:-
B) Y:- + RX RY + X:-
C) Y: + RX+ RY+ + X:
D) Y:- + RX+ RY + X:
E) RX+ R+ + X:
A) Y: + RX RY+ + X:-
B) Y:- + RX RY + X:-
C) Y: + RX+ RY+ + X:
D) Y:- + RX+ RY + X:
E) RX+ R+ + X:

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following would be most reactive in an SN2 reaction?
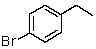
A)
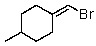
B)
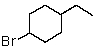
C)
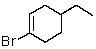
D)
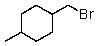
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
57
Identify the leaving group in the following reaction. 
A) C6H5S-
B) Na+
C) CH3CH2I
D) C6H5SCH2CH3
E) I-

A) C6H5S-
B) Na+
C) CH3CH2I
D) C6H5SCH2CH3
E) I-

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
58
Which is not a polar aprotic solvent?
A)
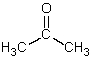
B)
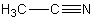
C)
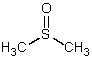
D)
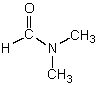
E)
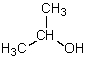
A)

B)
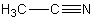
C)

D)

E)


Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
59
The relative nucleophilicities of species do not necessarily parallel the relative basicities of the same species because:
A) not all nucleophiles are bases,and vice versa.
B) experimental measurements of sufficient accuracy are not available to make the comparisons.
C) nucleophilicity is a thermodynamic matter;basicity is a matter of kinetics.
D) basicity is a thermodynamic matter;nucleophilicity is a matter of kinetics.
E) Actually,the relative values do parallel one another.
A) not all nucleophiles are bases,and vice versa.
B) experimental measurements of sufficient accuracy are not available to make the comparisons.
C) nucleophilicity is a thermodynamic matter;basicity is a matter of kinetics.
D) basicity is a thermodynamic matter;nucleophilicity is a matter of kinetics.
E) Actually,the relative values do parallel one another.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
60
Which is a polar aprotic solvent?
A) 2-methylhexane
B) CCl4
C) NH3(l)
D) CH3CH2CH2OCH2CH2CH3
E) 2-methyl-2-propanol
A) 2-methylhexane
B) CCl4
C) NH3(l)
D) CH3CH2CH2OCH2CH2CH3
E) 2-methyl-2-propanol

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
61
By analyzing the starting material and the product(s),the following reaction is possibly an example of what type of mechanism? 
A) SN1
B) SN2
C) E1
D) E2
E) None of the above

A) SN1
B) SN2
C) E1
D) E2
E) None of the above

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
62
What would be the major product(s)of the following reaction? 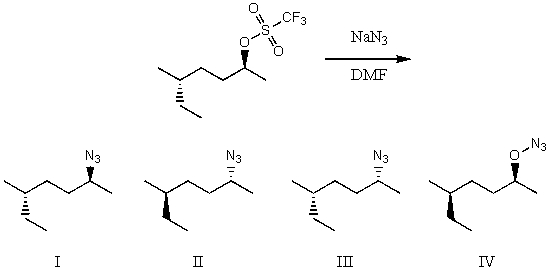
A) I
B) II
C) III
D) IV
E) None of the above

A) I
B) II
C) III
D) IV
E) None of the above

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
63
Elimination reactions are favored over nucleophilic substitution reactions:
A) at high temperatures.
B) when tert-butoxide ion is used.
C) when 3 alkyl halides are used as substrates.
D) when nucleophiles are used which are strong bases and the substrate is a 2 alkyl halide.
E) in all of these cases.
A) at high temperatures.
B) when tert-butoxide ion is used.
C) when 3 alkyl halides are used as substrates.
D) when nucleophiles are used which are strong bases and the substrate is a 2 alkyl halide.
E) in all of these cases.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
64
What would you expect to be the chief organic product(s)when tert-butyl bromide reacts with sodium acetylide,i.e. , 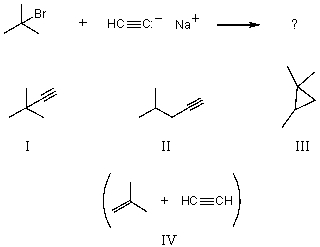
A) I
B) II
C) III
D) IV
E) None of these

A) I
B) II
C) III
D) IV
E) None of these

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
65
What would be the major product of the following reaction? 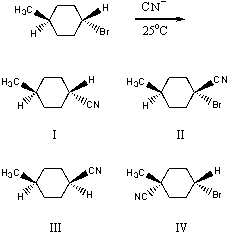
A) I
B) II
C) III
D) IV
E) Equal amounts of I and III

A) I
B) II
C) III
D) IV
E) Equal amounts of I and III

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
66
The major product(s)of the following reaction is(are): 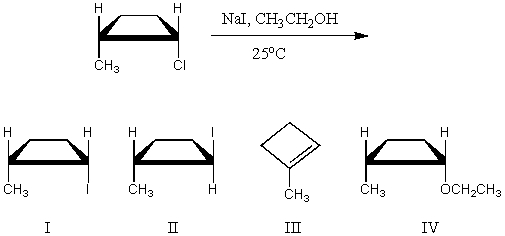
A) I
B) II
C) III
D) IV
E) Equal amounts of I and II

A) I
B) II
C) III
D) IV
E) Equal amounts of I and II

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
67
Which alkyl halide,when treated with sodium ethoxide in ethanol,would afford a product mixture consisting of more than one elimination product?
A) 1-bromo-3,3-dimethylpentane
B) 1-bromo-2,3-dimethylpentane
C) 2-bromo-3,4-dimethylpentane
D) 2-bromo-3,3-dimethylpentane
E) None of the above would yield more than one elimination product.
A) 1-bromo-3,3-dimethylpentane
B) 1-bromo-2,3-dimethylpentane
C) 2-bromo-3,4-dimethylpentane
D) 2-bromo-3,3-dimethylpentane
E) None of the above would yield more than one elimination product.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
68
By analyzing the starting material and the product,the following reaction is possibly an example of what type of mechanism(s)? 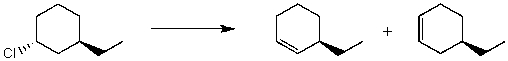
A) SN1
B) SN2
C) E1
D) E2
E) More than one of the above

A) SN1
B) SN2
C) E1
D) E2
E) More than one of the above

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
69
What would be the major product(s)of the following reaction? 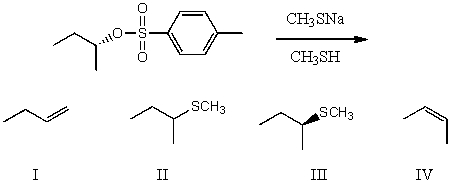
A) I
B) II
C) III
D) IV
E) More than one of the above

A) I
B) II
C) III
D) IV
E) More than one of the above

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
70
What would be the major product(s)of the following reaction? 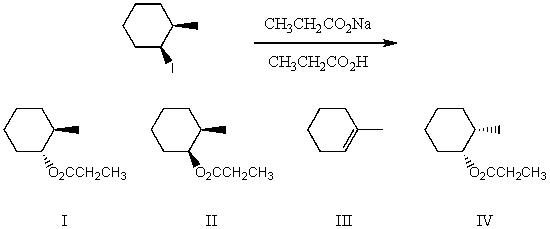
A) I
B) II
C) III
D) IV
E) None of the above

A) I
B) II
C) III
D) IV
E) None of the above

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
71
What major product(s)are likely to be obtained from the following reaction? 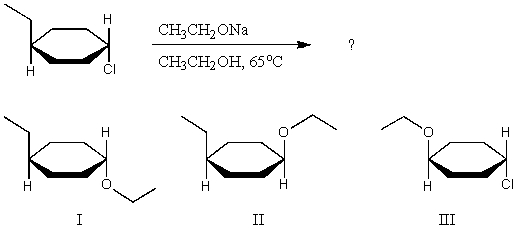
A) I,by predominantly SN2
B) II,by predominantly SN2
C) An equimolar mixture of I and II,by predominantly SN1.
D) III,by substitution of the alkyl group,rather than substitution of the chloro group.
E) Actually,none of these products are likely to be obtained as major products,because elimination will probably predominate,leading to the formation of an alkene.

A) I,by predominantly SN2
B) II,by predominantly SN2
C) An equimolar mixture of I and II,by predominantly SN1.
D) III,by substitution of the alkyl group,rather than substitution of the chloro group.
E) Actually,none of these products are likely to be obtained as major products,because elimination will probably predominate,leading to the formation of an alkene.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
72
Reaction of sodium ethoxide with 1-bromopentane at 30 C yields primarily:
A) CH3CH2CH2CH=CH2
B) CH3CH2CH=CHCH3
C) CH3CH2CH2CH2CH3
D) CH3CH2CH2CH2CH2OH
E) CH3CH2CH2CH2CH2OCH2CH3
A) CH3CH2CH2CH=CH2
B) CH3CH2CH=CHCH3
C) CH3CH2CH2CH2CH3
D) CH3CH2CH2CH2CH2OH
E) CH3CH2CH2CH2CH2OCH2CH3

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
73
What would be the major product(s)of the following reaction? 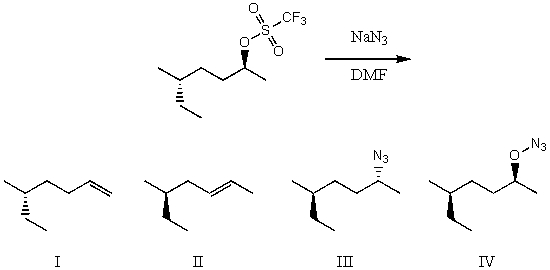
A) I
B) II
C) III
D) IV
E) None of the above

A) I
B) II
C) III
D) IV
E) None of the above

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
74
The product(s)for the following reaction would mainly be dictated by which mechanism? 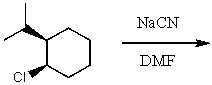
A) SN1
B) SN2
C) E1
D) E2
E) None of the above

A) SN1
B) SN2
C) E1
D) E2
E) None of the above

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
75
By analyzing the starting material and the product(s)the following reaction is an example of what type of mechanism? 
A) SN1
B) SN2
C) E1
D) E2
E) None of the above

A) SN1
B) SN2
C) E1
D) E2
E) None of the above

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
76
Which nucleophilic substitution reaction is not likely to occur?
A) I - + CH3CH2-Cl CH3CH2-I + Cl -
B) I - + CH3CH2-Br CH3CH2-I + Br -
C) I - + CH3CH2-OH CH3CH2-I + OH -
D) CH3O - + CH3CH2-Br CH3CH2-OCH3 + Br -
E) OH - + CH3CH2-Cl CH3CH2-OH + Cl -
A) I - + CH3CH2-Cl CH3CH2-I + Cl -
B) I - + CH3CH2-Br CH3CH2-I + Br -
C) I - + CH3CH2-OH CH3CH2-I + OH -
D) CH3O - + CH3CH2-Br CH3CH2-OCH3 + Br -
E) OH - + CH3CH2-Cl CH3CH2-OH + Cl -

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
77
Heating an alcoholic solution of sodium hydroxide and 1-bromopentane at 60 C yields primarily:
A)

B)
C)

D)

E)

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
78
What would be the major product(s)of the following reaction? 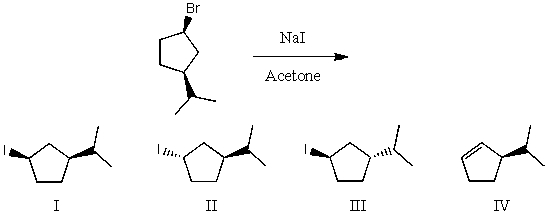
A) I
B) II
C) III
D) IV
E) None of the above

A) I
B) II
C) III
D) IV
E) None of the above

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
79
What would be the major product of the following reaction? 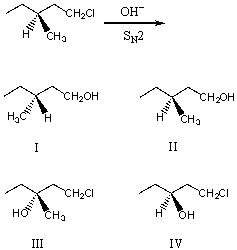
A) I
B) II
C) III
D) IV
E) An equimolar mixture of I and II

A) I
B) II
C) III
D) IV
E) An equimolar mixture of I and II

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
80
What would you expect to be the chief organic product(s)when 2-bromo-2-methylpentane react(s)with sodium propynide,i.e. , 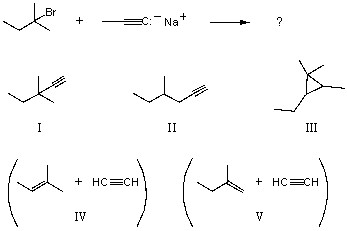
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck


