Exam 6: Ionic Reactions - Nucleophilic Substitution and Elimination Reactions of Alkyl Halides
Exam 1: Carbon Compounds and Chemical Bonds134 Questions
Exam 2: Representative Carbon Compounds: Functional Groups, Intermolecular Forces, and Infrared Ir Spectroscopy114 Questions
Exam 3: An Introduction to Organic Reactions: Acids and Bases47 Questions
Exam 4: Alkanes: Nomenclature, Conformational Analysis, and an Introduction to Synthesis125 Questions
Exam 5: Stereochemistry: Chiral Molecules150 Questions
Exam 6: Ionic Reactions - Nucleophilic Substitution and Elimination Reactions of Alkyl Halides146 Questions
Exam 7: Alkenes and Alkynes I: Properties and Synthesis, Elimination Reactions of Alkyl Halides99 Questions
Exam 8: Alkenes and Alkynes Ii: Addition Reactions140 Questions
Exam 9: Nuclear Magnetic Resonance and Mass Spectrometry: Tools for Structure Determination94 Questions
Exam 10: Radical Reactions114 Questions
Exam 11: Alcohols and Ethers172 Questions
Exam 12: Alcohols From Carbonyl Compounds Oxidation-Reduction and Organometallic Compounds147 Questions
Exam 13: Conjugated Unsaturated Systems166 Questions
Exam 14: Aromatic Compounds151 Questions
Exam 15: Reactions of Aromatic Compounds173 Questions
Exam 16: Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group165 Questions
Exam 17: Aldehydes and Ketones Ii Aldol Reactions131 Questions
Exam 18: Carboxylic Acids and Their Derivatives Nucleophilic Addition - Elimination at the Acyl Carbon124 Questions
Exam 19: Synthesis and Reactions of Beta-Dicarbonyl Compounds: More Chemistry of Enolate Ions131 Questions
Exam 20: Amines148 Questions
Exam 21: Phenols and Aryl Halides: Nucleophilic Aromatic Substitution87 Questions
Exam 22: Carbohydrates104 Questions
Exam 23: Lipids99 Questions
Exam 24: Amino Acids and Proteins94 Questions
Exam 25: Nucleic Acids and Protein Synthesis89 Questions
Select questions type
Select the potential energy diagram that represents a two-step endothermic (endergonic)reaction. 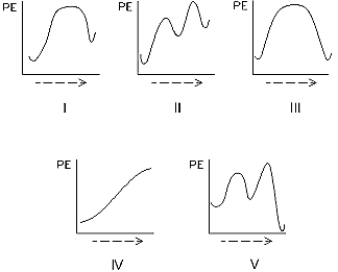
Free
(Multiple Choice)
4.9/5  (48)
(48)
Correct Answer:
B
Which of the following is not a good leaving group?
Free
(Multiple Choice)
4.9/5  (38)
(38)
Correct Answer:
A
An increase in the temperature at which a reaction is carried out increases:
Free
(Multiple Choice)
4.9/5  (36)
(36)
Correct Answer:
D
Consider the substitution reaction that takes place when (R)-3-bromo-3-methylhexane is treated with methanol.Which of the following would be true?
(Multiple Choice)
4.9/5  (29)
(29)
A true statement about the transition state(s)of an SN2 reaction is:
(Multiple Choice)
4.8/5  (30)
(30)
Which alkyl halide would be most reactive in an SN1 reaction? 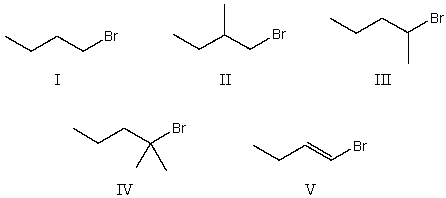
(Multiple Choice)
4.9/5  (34)
(34)
The product(s)for the following reaction would mainly be dictated by which mechanism? 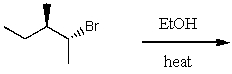
(Multiple Choice)
4.9/5  (47)
(47)
The major product(s)for the following reaction would mainly be dictated by which mechanism? 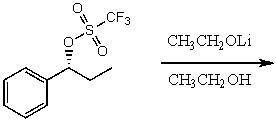
(Multiple Choice)
4.9/5  (36)
(36)
What would be the major product(s)of the following reaction? 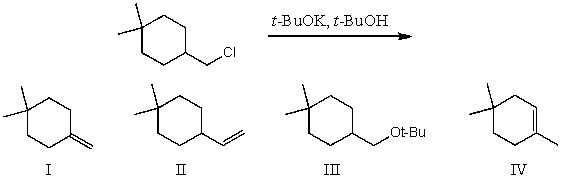
(Multiple Choice)
4.9/5  (31)
(31)
Treating (CH3)3C-Cl with a mixture of H2O and CH3OH at room temperature would yield:
(Multiple Choice)
4.7/5  (29)
(29)
Consider the SN2 reaction of butyl bromide with OH- ion. CH3CH2CH2CH2Br + OH- CH3CH2CH2CH2OH + Br-
Assuming no other changes,what effect on the rate would result from simultaneously doubling the concentrations of both butyl bromide and OH- ion?
(Multiple Choice)
4.9/5  (41)
(41)
What would be the major product(s)of the following reaction? 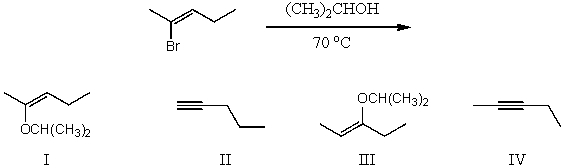
(Multiple Choice)
4.9/5  (45)
(45)
The product(s)for the following reaction would mainly be dictated by which mechanism? 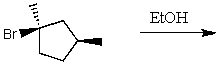
(Multiple Choice)
4.8/5  (39)
(39)
If 0.10 mol of HSCH2CH2OH reacts at 25 C,sequentially,with 0.20 mol of NaH,0.10 mol of CH3CH2Br and H2O,which is the major product?
(Multiple Choice)
4.8/5  (36)
(36)
What would be the major product(s)of the following reaction? 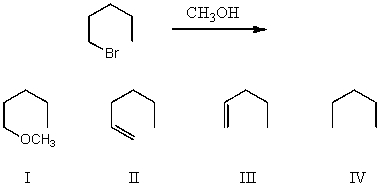
(Multiple Choice)
4.8/5  (48)
(48)
Which alkyl halide would you expect to undergo an SN2 reaction most slowly?
(Multiple Choice)
4.8/5  (30)
(30)
Showing 1 - 20 of 146
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)