Deck 14: Wave Particle Duality and Quantum Physics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/153
Play
Full screen (f)
Deck 14: Wave Particle Duality and Quantum Physics
1
If the frequency of light causing photoemission of electrons is doubled, the kinetic energy of the ejected electrons
A) increases by a factor of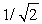
B) doubles.
C) decreases by a factor of 2.
D) increases by a factor less than two.
E) increases by a factor more than two.
A) increases by a factor of
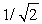
B) doubles.
C) decreases by a factor of 2.
D) increases by a factor less than two.
E) increases by a factor more than two.
increases by a factor more than two.
2
Light of wavelength 400 nm is incident on a certain metal. The stopping potential for the emitted electrons is measured to be 1.2 V. What is the work function of this metal? (Planck's constant h = 6.626 * 10-34 J · s = 4.136 * 10-15 eV s.)
A) 4.3 eV
B) 3.1 eV
C) 1.9 eV
D) 1.2 eV
E) 0.95 eV
A) 4.3 eV
B) 3.1 eV
C) 1.9 eV
D) 1.2 eV
E) 0.95 eV
1.9 eV
3
Which of the following statements about Maxwell's theory of electromagnetism is false?
A) The theory shows that light travels as transverse waves.
B) The theory shows that the speed of light is c in vacuum.
C) The theory shows that light travels as photons.
D) The theory shows that light consists of electric and magnetic waves.
E) The theory shows that light does not depend on a medium for it to travel.
A) The theory shows that light travels as transverse waves.
B) The theory shows that the speed of light is c in vacuum.
C) The theory shows that light travels as photons.
D) The theory shows that light consists of electric and magnetic waves.
E) The theory shows that light does not depend on a medium for it to travel.
The theory shows that light travels as photons.
4
Which of the following statements concerning particles and waves is/are correct?
A) Uncharged particles travel in straight lines until they collide with something.
B) When two particles meet in space, they never produce an interference pattern.
C) When a large number of small particles exchange a small amount of energy, the exchange cannot be distinguished from that of a wave.
D) When two waves of equal intensity and originating from coherent sources meet in space, the resulting wave can have an intensity anywhere from zero to four times the intensity of each of the original waves.
E) All of the above are correct.
A) Uncharged particles travel in straight lines until they collide with something.
B) When two particles meet in space, they never produce an interference pattern.
C) When a large number of small particles exchange a small amount of energy, the exchange cannot be distinguished from that of a wave.
D) When two waves of equal intensity and originating from coherent sources meet in space, the resulting wave can have an intensity anywhere from zero to four times the intensity of each of the original waves.
E) All of the above are correct.

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
5
The wavelength of red light is closest to
A) 100 nm
B) 300 nm
C) 500 nm
D) 700 nm
E) 900 nm
A) 100 nm
B) 300 nm
C) 500 nm
D) 700 nm
E) 900 nm

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
6
Photons with an energy of 7.52 eV strike a material that has a work function of 4.22 eV. The maximum kinetic energy of the electron emitted from this material is
A) 7.5 eV
B) 12 eV
C) 3.3 eV
D) 0.98 eV
E) No electrons are ejected by these photons.
A) 7.5 eV
B) 12 eV
C) 3.3 eV
D) 0.98 eV
E) No electrons are ejected by these photons.

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
7
Estimate the number of photons emitted by the Sun in a second. The power output from the Sun is 4 *1026 W and assume that the average wavelength of each photon is 550 nm.
A) 1026
B) 1045
C) 1023
D) 1028
E) none of the above
A) 1026
B) 1045
C) 1023
D) 1028
E) none of the above

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
8
The visible portion of the electromagnetic spectrum extends from
A) 200 nm to 500 nm.
B) 300 nm to 600 nm.
C) 400 nm to 700 nm.
D) 500 nm to 800 nm.
E) 600 nm to 900 nm.
A) 200 nm to 500 nm.
B) 300 nm to 600 nm.
C) 400 nm to 700 nm.
D) 500 nm to 800 nm.
E) 600 nm to 900 nm.

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
9
In the photoelectric effect, the work function depends on the
A) incident wavelength.
B) applied voltage.
C) light intensity.
D) metal that the light strikes.
E) current.
A) incident wavelength.
B) applied voltage.
C) light intensity.
D) metal that the light strikes.
E) current.

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
10
The velocity of escape of photoelectrons
A) increases with increasing frequency of the incident light.
B) decreases with increasing frequency of the incident light.
C) is independent of the frequency of the incident light.
D) is directly proportional to the intensity of the incident light.
E) depends only on the intensity of the incident light.
A) increases with increasing frequency of the incident light.
B) decreases with increasing frequency of the incident light.
C) is independent of the frequency of the incident light.
D) is directly proportional to the intensity of the incident light.
E) depends only on the intensity of the incident light.

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
11
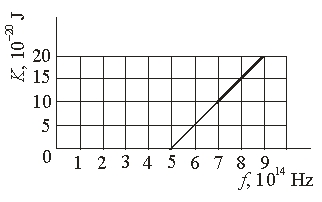 The graph shows the kinetic energy of photoelectrons ejected from a certain metal as a function of the light incident upon the metal. The work function of this metal is
The graph shows the kinetic energy of photoelectrons ejected from a certain metal as a function of the light incident upon the metal. The work function of this metal isA) 33* 10-20 J
B) 20 *10-20 J
C) 10 * 10-20 J
D) 5 * 10-20 J
E) None of these is correct.

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
12
In a photoelectric experiment, the threshold frequency for a material is 3.2 * 1014 Hz. An electron ejected from this surface by a photon of frequency 9.4 * 1014 Hz can be stopped by a stopping potential of
A) 8.5 mV
B) 1.6 V
C) 0.26 nV
D) 2.6 V
E) 3.6 kV
A) 8.5 mV
B) 1.6 V
C) 0.26 nV
D) 2.6 V
E) 3.6 kV

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
13
The wavelength of blue light is closest to
A) 400 nm
B) 500 nm
C) 600 nm
D) 700 nm
E) 800 nm
A) 400 nm
B) 500 nm
C) 600 nm
D) 700 nm
E) 800 nm

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
14
In Young's experiment,
A) each slit acts as a line source.
B) an interference pattern is observed on a screen placed behind the slits.
C) interference maxima occur at angles such that the path difference is an integral number of wavelengths.
D) interference minima occur when the path difference is one half wavelength or any odd number of half wavelengths.
E) all of the above are correct.
A) each slit acts as a line source.
B) an interference pattern is observed on a screen placed behind the slits.
C) interference maxima occur at angles such that the path difference is an integral number of wavelengths.
D) interference minima occur when the path difference is one half wavelength or any odd number of half wavelengths.
E) all of the above are correct.

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
15
The maximum wavelength for the photoemission of electrons from a metal surface depends on
A) applied voltage.
B) light intensity.
C) the metal that the light strikes.
D) current.
E) None of these is correct.
A) applied voltage.
B) light intensity.
C) the metal that the light strikes.
D) current.
E) None of these is correct.

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
16
The maximum kinetic energy of electrons ejected from barium (whose work function is 2.50 eV) when it is illuminated by light of wavelength 350 nm is
A) 0.20 eV
B) 0.41 eV
C) 0.63 eV
D) 0.95 eV
E) 1.05 eV
A) 0.20 eV
B) 0.41 eV
C) 0.63 eV
D) 0.95 eV
E) 1.05 eV

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
17
The work function for tungsten is 4.58 eV. What is the kinetic energy of electrons emitted when light of wavelength 400 nm is incident on a tungsten surface? (Planck's constant h = 6.626 * 10-34 J · s = 4.136 * 10-15 eV · s.)
A) 0.74 eV
B) 1.5 eV
C) 7.7 eV
D) 2.9 eV
E) no electrons are emitted
A) 0.74 eV
B) 1.5 eV
C) 7.7 eV
D) 2.9 eV
E) no electrons are emitted

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
18
Potassium has a work function of 2.3 eV for photoelectric emission. Which of the following wavelengths is the longest wavelength for which photoemission occurs?
A) 400 nm
B) 450 nm
C) 500 nm
D) 540 nm
E) 600 nm
A) 400 nm
B) 450 nm
C) 500 nm
D) 540 nm
E) 600 nm

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following experiment(s) illustrates the particle nature of light?
A) Young's double slit experiment
B) the photoelectric effect
C) Compton scattering
D) (A) and (B)
E) (B) and (C)
A) Young's double slit experiment
B) the photoelectric effect
C) Compton scattering
D) (A) and (B)
E) (B) and (C)

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
20
Albert Einstein was awarded the Nobel Prize for his
A) theory of special relativity.
B) theory of general relativity.
C) explanation of Brownian motion.
D) explanation of the Michelson-Morley experiment.
E) explanation of the photoelectric effect.
A) theory of special relativity.
B) theory of general relativity.
C) explanation of Brownian motion.
D) explanation of the Michelson-Morley experiment.
E) explanation of the photoelectric effect.

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
21
Use the following figure for the next two questions. 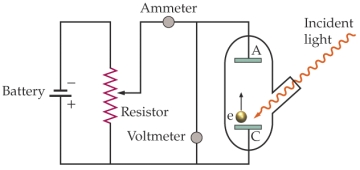 Apparatus for studying the photoelectric effect
Apparatus for studying the photoelectric effect
-The work function of the material being investigated is 3.5 * 10-19 J. The battery is set at 1.5 V. What is the longest wavelength of light needed to produce an electric current from the cathode (C) to the anode (A)?
A) 337 nm
B) 354 nm
C) 620 nm
D) 827 nm
E) None of these is correct.
 Apparatus for studying the photoelectric effect
Apparatus for studying the photoelectric effect-The work function of the material being investigated is 3.5 * 10-19 J. The battery is set at 1.5 V. What is the longest wavelength of light needed to produce an electric current from the cathode (C) to the anode (A)?
A) 337 nm
B) 354 nm
C) 620 nm
D) 827 nm
E) None of these is correct.

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following formulas has the correct units for the linear momentum of a photon?
A) hc/
B) c/n
C) l/
D) /h
E) None of these is correct.
A) hc/
B) c/n
C) l/
D) /h
E) None of these is correct.

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
23
The photoelectric threshold of a certain metal is 310 nm. The maximum kinetic energy of electrons ejected from the surface of the metal by ultraviolet light of wavelength
200 nm is
A) 2.22 eV
B) 2.60 eV
C) 10.2 eV
D) 12.3 eV
E) none of these because no electrons are ejected
200 nm is
A) 2.22 eV
B) 2.60 eV
C) 10.2 eV
D) 12.3 eV
E) none of these because no electrons are ejected

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
24
What is the momentum (in SI units) of a photon of wavelength = 410 nm? (Planck's constant h = 6.626 *10-34 J·s)
A) 1.62 *-27 kg m/s
B) 2.45* 10-27 kg m/s
C) 3.23 *10-27 kg m/s
D) 4.67 * 10-27 kg m/s
E) 5.29 * 10-27 kg m/s
A) 1.62 *-27 kg m/s
B) 2.45* 10-27 kg m/s
C) 3.23 *10-27 kg m/s
D) 4.67 * 10-27 kg m/s
E) 5.29 * 10-27 kg m/s

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
25
Use the following figure for the next two questions.  Apparatus for studying the photoelectric effect
Apparatus for studying the photoelectric effect
-The work function of the material being investigated is 3.8 * 10-19 J. Light of a wavelength 350 nm is incident on the material. What is the lowest voltage needed between the cathode (C) and the anode (A) to stop any electrons ejected from the cathode from reaching the anode?
A) 2.37 V
B) 1.17 V
C) 3.54 V
D) 4.71 V
E) None of these is correct.
 Apparatus for studying the photoelectric effect
Apparatus for studying the photoelectric effect-The work function of the material being investigated is 3.8 * 10-19 J. Light of a wavelength 350 nm is incident on the material. What is the lowest voltage needed between the cathode (C) and the anode (A) to stop any electrons ejected from the cathode from reaching the anode?
A) 2.37 V
B) 1.17 V
C) 3.54 V
D) 4.71 V
E) None of these is correct.

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
26
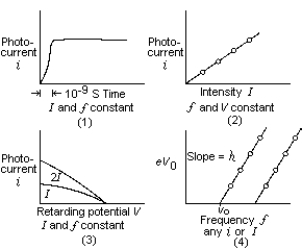 Classical electromagnetic theory was successful in explaining or predicting only one of the characteristics or experimental results of photoelectric emission. These graphs illustrate these main experimental results. Which one is in agreement with predictions of classical electromagnetic theory?
Classical electromagnetic theory was successful in explaining or predicting only one of the characteristics or experimental results of photoelectric emission. These graphs illustrate these main experimental results. Which one is in agreement with predictions of classical electromagnetic theory?A) 1
B) 2
C) 3
D) 4
E) None of these is correct.

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
27
An X-ray photon scattering off an electron imparts some of its energy to the electron (the Compton effect). Which of the following statements is true?
A) The wavelength of the scattered photon is unchanged.
B) The wavelength of the scattered photon is decreased.
C) The wavelength of the scattered photon is increased.
D) The scattered photon gains speed.
E) The scattered photon is slowed.
A) The wavelength of the scattered photon is unchanged.
B) The wavelength of the scattered photon is decreased.
C) The wavelength of the scattered photon is increased.
D) The scattered photon gains speed.
E) The scattered photon is slowed.

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
28
Light falling on the surface of a metal such as cesium can liberate electrons from the metal. The kinetic energy of electrons emitted from a metal can be increased by
A) using light of higher frequency.
B) using light of lower frequency.
C) increasing the intensity of the incident light.
D) using a metal with a greater work function.
E) None of these is correct.
A) using light of higher frequency.
B) using light of lower frequency.
C) increasing the intensity of the incident light.
D) using a metal with a greater work function.
E) None of these is correct.

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
29
If the work function of thoriated tungsten is 4 *10-19 J, the longest wavelength of light that will cause photoelectrons to be emitted is approximately
A) 880 nm
B) 400 nm
C) 495 nm
D) 700 nm
E) 181 nm
A) 880 nm
B) 400 nm
C) 495 nm
D) 700 nm
E) 181 nm

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
30
The man whose name is most closely associated with the explanation of the photoelectric effect is
A) M. Planck.
B) E. Fermi.
C) N. Bohr.
D) E. Rutherford.
E) A. Einstein.
A) M. Planck.
B) E. Fermi.
C) N. Bohr.
D) E. Rutherford.
E) A. Einstein.

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
31
The maximum kinetic energy of photoelectrons produced in the photoelectric effect depends directly on the
A) frequency of the incident photons.
B) intensity of the incident photons.
C) area of the metal surface from which the photoelectrons are released.
D) thickness of the metal.
E) photoelectric current.
A) frequency of the incident photons.
B) intensity of the incident photons.
C) area of the metal surface from which the photoelectrons are released.
D) thickness of the metal.
E) photoelectric current.

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
32
What is the momentum (in SI units) of a photon of wavelength = 560 nm? (Planck's constant h = 6.626 *10-34 J·s.)
A) 3.7 *10-42 kg · m/s
B) 8.4 * 10-25 kg · m/s
C) 1.2 * 10-30 kg · m/s
D) 1.2 *10-27 kg · m/s
E) 3.6 * 10-27 kg · m/s
A) 3.7 *10-42 kg · m/s
B) 8.4 * 10-25 kg · m/s
C) 1.2 * 10-30 kg · m/s
D) 1.2 *10-27 kg · m/s
E) 3.6 * 10-27 kg · m/s

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
33
A Compton-scattered X-ray photon has less energy than the incident photon. The scattered photon therefore
A) has the same wavelength as the incident photon.
B) has a longer wavelength than the incident photon.
C) has a shorter wavelength than the incident photon.
D) cannot be assigned a wavelength.
E) has none of these wavelengths.
A) has the same wavelength as the incident photon.
B) has a longer wavelength than the incident photon.
C) has a shorter wavelength than the incident photon.
D) cannot be assigned a wavelength.
E) has none of these wavelengths.

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
34
When a certain X ray is Compton scattered at right angles to its initial direction, the shift in its wavelength is c = 2.4 pm (1 picometer = 10-12 m). If the wavelength of this X ray is 15.4 pm, the wavelength of the scattered X ray must be closest to
A) 2.4 pm
B) 0.15 pm
C) 18 pm
D) 13 pm
E) 170 pm
A) 2.4 pm
B) 0.15 pm
C) 18 pm
D) 13 pm
E) 170 pm

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
35
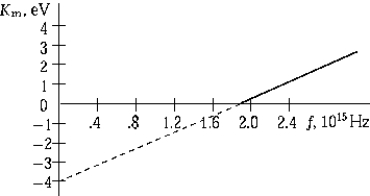 The graph shows the maximum kinetic energy of electrons emitted by a photosensitive surface as a function of the frequency of the incident radiation. The slope of this curve represents
The graph shows the maximum kinetic energy of electrons emitted by a photosensitive surface as a function of the frequency of the incident radiation. The slope of this curve representsA) the intensity of the incident radiation.
B) the maximum kinetic energy.
C) the threshold frequency.
D) Planck's constant.
E) the stopping potential.

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
36
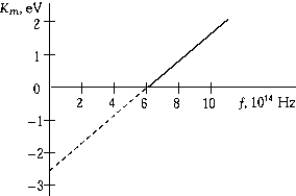 The graphs shows the maximum kinetic energy of electrons emitted from a metal as a function of the frequency of the incident light. The work function of the metal is
The graphs shows the maximum kinetic energy of electrons emitted from a metal as a function of the frequency of the incident light. The work function of the metal isA) 6.0 eV
B) 4.0 eV
C) 2.5 eV
D) 0.4 eV
E) 4 * 10-19 eV

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
37
Calculate the photon energy for light of wavelength 500 nm. (Planck's constant h = 6.626 * 10-34 J·s)
A) 1.24 eV
B) 1.98 eV
C) 2.24 eV
D) 2.48 eV
E) 2.98 eV
A) 1.24 eV
B) 1.98 eV
C) 2.24 eV
D) 2.48 eV
E) 2.98 eV

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
38
What is the momentum (in SI units) of a photon of wavelength = 480 nm? (Planck's constant h = 6.626 * 10-34 J·s)
A) 7.24 * 10-27 kg m/s
B) 6.21 * 10-27 kg m/s
C) 4.14 *10-27 kg m/s
D) 2.76 * 10-27 kg m/s
E) 1.38 * 10-27 kg m/s
A) 7.24 * 10-27 kg m/s
B) 6.21 * 10-27 kg m/s
C) 4.14 *10-27 kg m/s
D) 2.76 * 10-27 kg m/s
E) 1.38 * 10-27 kg m/s

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
39
In the Compton effect,
A) an electron is stopped suddenly.
B) an X ray collides with an electron, the electron is ejected, and the X-ray photon ceases to exist.
C) a neutrino is produced in a nuclear decay.
D) an energetic photon collides with an electron, and both the electron and the photon are scattered.
E) None of these is correct.
A) an electron is stopped suddenly.
B) an X ray collides with an electron, the electron is ejected, and the X-ray photon ceases to exist.
C) a neutrino is produced in a nuclear decay.
D) an energetic photon collides with an electron, and both the electron and the photon are scattered.
E) None of these is correct.

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
40
Photon A has twice the energy of photon B. The ratio of the momentum of A to that of B is
A) 4
B) 2
C) 1
D) 1/2
E) 1/4
A) 4
B) 2
C) 1
D) 1/2
E) 1/4

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
41
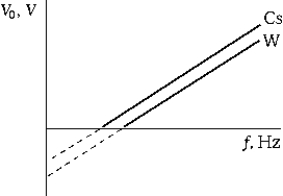 The graph shows the stopping potential for photoelectrons as a function of the frequency of the incident light for two different metals (Cs and W). Which of the following statements is true?
The graph shows the stopping potential for photoelectrons as a function of the frequency of the incident light for two different metals (Cs and W). Which of the following statements is true?A) Because the intercepts are different, Planck's constant is not constant.
B) For a given frequency of incident light, the photoelectrons from W are more energetic than those from Cs.
C) The maximum kinetic energy of the photoelectrons increases as the frequency of the incident light increases.
D) The stopping potentials for all metals are the same.
E) The threshold frequency for Cs is greater than that for W.

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
42
In a Compton scattering experiment, an 8.00-MeV incident photon is scattered at an angle of 15º with energy of 5.20 MeV. The kinetic energy of the recoil electron is
A) 2.80 MeV
B) 2.08 MeV
C) 5.20 MeV
D) 7.28 MeV
E) 800 MeV
A) 2.80 MeV
B) 2.08 MeV
C) 5.20 MeV
D) 7.28 MeV
E) 800 MeV

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
43
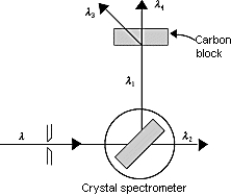 The figure shows X-rays incident on a crystal spectrometer that are reflected from the crystal and used for Compton scattering in a carbon block. Of the wavelengths shown, 3 ; thus, which of the following expressions is true?
The figure shows X-rays incident on a crystal spectrometer that are reflected from the crystal and used for Compton scattering in a carbon block. Of the wavelengths shown, 3 ; thus, which of the following expressions is true?A) 1 = 2 = 3 = 4
B) = 1 > 3
C) < 2 < 1 < 4
D) 3 > 1 = 4
E) 4 > 2 >

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
44
An X-ray photon of wavelength 0.10 nm is Compton-scattered from a free electron. The greatest observed shift in wavelength, as seen in the scattered photon, is
A) 0.0012 nm
B) 0.0024 nm
C) 0.0048 nm
D) 0.18 nm
E) 0.10 nm
A) 0.0012 nm
B) 0.0024 nm
C) 0.0048 nm
D) 0.18 nm
E) 0.10 nm

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
45
A gamma-ray photon of energy 1.00 MeV scatters off an electron at an angle perpendicular to its original direction. Calculate the kinetic energy of the recoiling electron.
A) 489 keV
B) 511 keV
C) 663 keV
D) 253 keV
E) 337 keV
A) 489 keV
B) 511 keV
C) 663 keV
D) 253 keV
E) 337 keV

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
46
When a surface is illuminated with light of wavelength 500 nm, the maximum speed of the photoelectrons is 300 km/s. When the same surface is illuminated by light of half this wavelength what is the maximum speed of the photoelectrons in this case?
A) 680 km/s
B) 1280 km/s
C) 885 km/s
D) 980 km/s
E) 14800 km/s
A) 680 km/s
B) 1280 km/s
C) 885 km/s
D) 980 km/s
E) 14800 km/s

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
47
Microwaves range in wavelength from about 2.0 * 10-2 cm to about 5.0 cm. What is the maximum energy in eV that a microwave may have?
A) 0.025 meV
B) 6.2 meV
C) 2.5 eV
D) 6.2 eV
E) 0.62 meV
A) 0.025 meV
B) 6.2 meV
C) 2.5 eV
D) 6.2 eV
E) 0.62 meV

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
48
The work functions for two different metals A and B, are 3.23 eV and 5.33 eV, respectively. If photons of 220 nm are incident on the two metals, calculate the difference in energy of the fastest photoelectrons from metal A and metal B.
A) 0.69 eV
B) 0.0 eV
C) 2.10 eV
D) 1.72 eV
E) 2.41 eV
A) 0.69 eV
B) 0.0 eV
C) 2.10 eV
D) 1.72 eV
E) 2.41 eV

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
49
The dissociation energy is the energy required to separate the two atoms in a diatomic molecule in their ground-state. If the dissociation energy of molecular oxygen is 7.2 eV, then calculate the maximum wavelength of light from the Sun that can break apart atmospheric O2.
A) 1.7 * 10-7 nm
B) 1.7 * 10-8 m
C) 1.7 *10-9 m
D) 1.7 *10-7 m
E) 1.7 * 102 m
A) 1.7 * 10-7 nm
B) 1.7 * 10-8 m
C) 1.7 *10-9 m
D) 1.7 *10-7 m
E) 1.7 * 102 m

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
50
Photons of wavelength 0.00150 nm undergo Compton collisions with free electrons. What is the energy of the scattered photons whose angle of scattering is 60º?
A) 13.3 * 10-14 J
B) 3.27 *10-14 J
C) 28.0 * 10-14 J
D) 7.36 * 10-14 J
E) 2.14 *10-14 J
A) 13.3 * 10-14 J
B) 3.27 *10-14 J
C) 28.0 * 10-14 J
D) 7.36 * 10-14 J
E) 2.14 *10-14 J

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
51
Photons of wavelength 0.00150 nm undergo Compton collisions with free electrons. What is the energy of the scattered photons whose angle of scattering is 45º?
A) 13.3 *10-14 J
B) 9.00* 10-14 J
C) 28.0 *10-14 J
D) 18.0 * 10-14 J
E) 2.14 * 10-14 J
A) 13.3 *10-14 J
B) 9.00* 10-14 J
C) 28.0 *10-14 J
D) 18.0 * 10-14 J
E) 2.14 * 10-14 J

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
52
The work function for a metal is 5.58 eV. What is the threshold wavelength for the photoelectric effect of incident photons?
A) 2.22 *10-7 m
B) 2.22* 10-9 m
C) 4.50 * 106 m
D) 4.50 * 10-6 m
E) 2.22 * 10-11 m
A) 2.22 *10-7 m
B) 2.22* 10-9 m
C) 4.50 * 106 m
D) 4.50 * 10-6 m
E) 2.22 * 10-11 m

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
53
A gamma-ray photon is back scattered at an angle of 180° after a collision with an electron. If the scattered photon has energy 150 keV, what was the energy of the initial photon?
A) 303 keV
B) 363 keV
C) 95 keV
D) 427 keV
E) 393 keV
A) 303 keV
B) 363 keV
C) 95 keV
D) 427 keV
E) 393 keV

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
54
A gamma-ray photon of energy 800 keV scatters off an electron at an angle perpendicular to its original direction. It then scatters off a second electron such that this secondary scattered photon continues in the direction of the original photon. Calculate the difference in energy between the first and second recoiling electrons.
A) 606 keV
B) 194 keV
C) 488 keV
D) 118 keV
E) 370 keV
A) 606 keV
B) 194 keV
C) 488 keV
D) 118 keV
E) 370 keV

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
55
Calculate the photon energy for light of wavelength 600 nm. (Planck's constant h = 6.626 *10-34 J·s)
A) 1.24 eV
B) 1.98 eV
C) 2.07 eV
D) 2.48 eV
E) 2.98 eV
A) 1.24 eV
B) 1.98 eV
C) 2.07 eV
D) 2.48 eV
E) 2.98 eV

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
56
What is the shift in wavelength of a photon scattered off an electron at 160º?
A) 2.4 pm
B) 0.15 pm
C) 1.6 pm
D) 1.2 pm
E) 4.7 pm
A) 2.4 pm
B) 0.15 pm
C) 1.6 pm
D) 1.2 pm
E) 4.7 pm

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
57
In Compton scattering of X rays by electrons, the maximum shift in the X-ray wavelength occurs when the scattering angle is
A) 180º
B) 135º
C) 90º
D) 45º
E) 0º
A) 180º
B) 135º
C) 90º
D) 45º
E) 0º

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
58
Magnetic Resonance Imaging (MRI) is a much used noninvasive diagnostic technique in medicine. The radio frequency photons used have energies of about 10-7 eV. What wavelength does this correspond to?
A) 12 nm
B) 1.2 m
C) 12 m
D) 1.2 nm
E) 120 m
A) 12 nm
B) 1.2 m
C) 12 m
D) 1.2 nm
E) 120 m

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
59
A gamma-ray photon of energy 800 keV scatters off an electron at an angle perpendicular to its original direction. It then scatters off a second electron such that this secondary scattered photon continues in the direction of the original photon. Calculate the energy difference between the initial and final photon.
A) 682 keV
B) 194 keV
C) 606 keV
D) 118 keV
E) 312 keV
A) 682 keV
B) 194 keV
C) 606 keV
D) 118 keV
E) 312 keV

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
60
The pupil area of the human eye is ~ 1*10-5 m2. The minimum light intensity that the eye is sensitive to is ~ 1 *10-10 Wm-2. How many photons per second of wavelength 550 nm does this correspond to?
A) ~300 s-1
B) ~30000 s-1
C) ~3000 s-1
D) ~3 * 10-2 s-1
E) ~3 * 1012 s-1
A) ~300 s-1
B) ~30000 s-1
C) ~3000 s-1
D) ~3 * 10-2 s-1
E) ~3 * 1012 s-1

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
61
The speed of an electron whose de Broglie wavelength is 0.0010 m is approximately
A) 7.3 * 10-7 m/s
B) 7.3 * 10-4 m/s
C) 0.73 m/s
D) 0.51 km/s
E) 5.1 * 105 m/s
A) 7.3 * 10-7 m/s
B) 7.3 * 10-4 m/s
C) 0.73 m/s
D) 0.51 km/s
E) 5.1 * 105 m/s

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
62
The fact that particles have a wave nature was first verified by
A) the Heisenberg uncertainty principle.
B) Davisson and Germer's diffraction of electrons experiment.
C) blackbody radiation studies.
D) the Bohr theory of the atom.
E) the photoelectric effect.
A) the Heisenberg uncertainty principle.
B) Davisson and Germer's diffraction of electrons experiment.
C) blackbody radiation studies.
D) the Bohr theory of the atom.
E) the photoelectric effect.

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
63
An electron (me = 9.11 *10-31 kg) traveling with a velocity of 5.50 *106 m/s has a
De Broglie wavelength of approximately
A) 0.0710 nm
B) 0.130 nm
C) 0.180 nm
D) 0.230 nm
E) 0.550 nm
De Broglie wavelength of approximately
A) 0.0710 nm
B) 0.130 nm
C) 0.180 nm
D) 0.230 nm
E) 0.550 nm

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
64
Electrons do not exhibit wave properties as readily as light because electrons typically have much __________ momenta than light and hence much __________ wavelengths.
A) greater; longer
B) greater; shorter
C) lesser; longer
D) lesser; shorter
E) greater; the same
A) greater; longer
B) greater; shorter
C) lesser; longer
D) lesser; shorter
E) greater; the same

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
65
If a baseball, an electron, and a photon all have the same momentum, which has the longest wavelength?
A) baseball
B) electron
C) photon
D) all have the same wavelength
E) it depends on the energy of the photon
A) baseball
B) electron
C) photon
D) all have the same wavelength
E) it depends on the energy of the photon

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following particles has the longest wavelength?
A) a photon with a frequency of 3 *1019 Hz
B) a photon with an energy of 2 *10-13 J
C) an electron with a momentum of 6.6*10-21 kg · m/s
D) a proton with a momentum of 6.6 * 10-21 kg · m/s
E) all four have the same wavelength
A) a photon with a frequency of 3 *1019 Hz
B) a photon with an energy of 2 *10-13 J
C) an electron with a momentum of 6.6*10-21 kg · m/s
D) a proton with a momentum of 6.6 * 10-21 kg · m/s
E) all four have the same wavelength

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
67
Use the following figure for the next two problems. 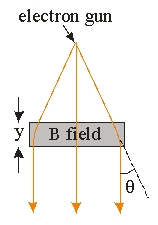
The electrons coming out of the electron gun in an electron microscope are being accelerated by a potential of 100 kV. The wavelength of the electrons is
A) 2.14 nm
B) 3.88 pm
C) 4.87 nm
D) 12.4 pm
E) 6.87 nm

The electrons coming out of the electron gun in an electron microscope are being accelerated by a potential of 100 kV. The wavelength of the electrons is
A) 2.14 nm
B) 3.88 pm
C) 4.87 nm
D) 12.4 pm
E) 6.87 nm

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
68
An electron (me = 9.11* 10-31 kg) traveling with a velocity of 1.1 *106 m/s has a
De Broglie wavelength of approximately
A) 0.66 nm
B) 0.78 nm
C) 0.89 nm
D) 0.97 nm
E) 1.2 nm
De Broglie wavelength of approximately
A) 0.66 nm
B) 0.78 nm
C) 0.89 nm
D) 0.97 nm
E) 1.2 nm

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
69
Use the following figure for the next two problems. 
-The electrons coming out of the electron gun in an electron microscope are being accelerated by a potential of 100 kV. The electrons then pass through a region of magnetic field of distance y = 8 cm which points perpendicularly to the direction of travel of the electron. What is the strength of the magnetic field needed to deflect the electron by an angle of = 25 ? (Ignore any relativistic effect. 1 Gauss = 10-4 T)
A) 120.1 Gauss
B) 30.4 Gauss
C) 21.8 Gauss
D) 14.6 Gauss
E) None of these is correct.

-The electrons coming out of the electron gun in an electron microscope are being accelerated by a potential of 100 kV. The electrons then pass through a region of magnetic field of distance y = 8 cm which points perpendicularly to the direction of travel of the electron. What is the strength of the magnetic field needed to deflect the electron by an angle of = 25 ? (Ignore any relativistic effect. 1 Gauss = 10-4 T)
A) 120.1 Gauss
B) 30.4 Gauss
C) 21.8 Gauss
D) 14.6 Gauss
E) None of these is correct.

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
70
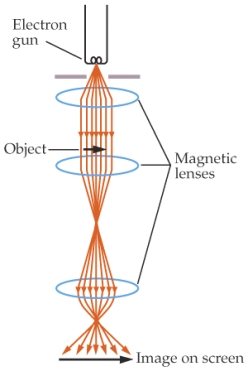 For the top magnetic lens, the direction of the magnetic field needed to deflect the electrons is
For the top magnetic lens, the direction of the magnetic field needed to deflect the electrons is 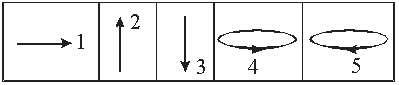
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
71
A proton has five times the momentum of an electron. If the electron has a de Broglie wavelength , then the de Broglie wavelength of the proton is
A)
B) 5
C) /5
D) 25
E) /25
A)
B) 5
C) /5
D) 25
E) /25

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
72
Suppose that the world could be changed so that the de Broglie wavelength of a 40-kg runner who goes 100 m in 10.0 s was 5 m. Assuming that all other constants remain the same, the value of Planck's constant would have to be
A) 0.80 J · s
B) 1.3 J · s
C) 0.76 J · s
D) 2.0 kJ · s
E) 0.20 MJ · s
A) 0.80 J · s
B) 1.3 J · s
C) 0.76 J · s
D) 2.0 kJ · s
E) 0.20 MJ · s

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
73
For an electron to have a de Broglie wavelength of 0.10 nm it must be accelerated through a potential difference of
A) 1.0 V
B) 0.51 MV
C) 0.15 kV
D) 5 kV
E) 0.94 kV
A) 1.0 V
B) 0.51 MV
C) 0.15 kV
D) 5 kV
E) 0.94 kV

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
74
The electron microscope is a welcome addition to the field of microscopy because electrons have a __________ wavelength than light, thereby increasing the __________ of the microscope.
A) longer; resolving power
B) longer; breadth of field
C) shorter; resolving power
D) longer; intensity
E) None of these is correct.
A) longer; resolving power
B) longer; breadth of field
C) shorter; resolving power
D) longer; intensity
E) None of these is correct.

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
75
An electron that is not localized in space is described by the wave function = A sin (kx - t). The kinetic energy of the electron is 10 keV. The value of is approximately
A) 1.5 *1019 s-1
B) 3.0 *1019 s-1
C) 4.5 * 1019 s-1
D) 1.5 * 1021 s-1
E) 3.0* 1021 s-1
A) 1.5 *1019 s-1
B) 3.0 *1019 s-1
C) 4.5 * 1019 s-1
D) 1.5 * 1021 s-1
E) 3.0* 1021 s-1

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
76
A 10.0-kg mass moving with a velocity of 100 m/s has a de Broglie wavelength of approximately
A) 1.98* 10-22 m
B) 1.51 *1036 m
C) 6.62 *10-33 m
D) 6.62 *10-37 m
E) 6.62 *10-31 m
A) 1.98* 10-22 m
B) 1.51 *1036 m
C) 6.62 *10-33 m
D) 6.62 *10-37 m
E) 6.62 *10-31 m

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
77
The wavelength of an electron is 1.33 nm. Its kinetic energy is approximately
A) 1.18 eV
B) 1.08 eV
C) 0.922 eV
D) 0.850 eV
E) 3.40 eV
A) 1.18 eV
B) 1.08 eV
C) 0.922 eV
D) 0.850 eV
E) 3.40 eV

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
78
An electron in the hydrogen atom makes a transition from an energy level of -1.51 eV to one of energy -3.40 eV. In this process a photon is emitted with what wavelength?
A) 1.89 nm
B) 656 nm
C) 456 nm
D) 821 nm
E) 365 nm
A) 1.89 nm
B) 656 nm
C) 456 nm
D) 821 nm
E) 365 nm

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
79
The Davisson and Germer experiment verified that
A) the velocity of light in a vacuum is a universal constant.
B) electrons have an intrinsic spin.
C) waves have a particle aspect.
D) electrons show wave characteristics.
E) the neutron does not have a charge.
A) the velocity of light in a vacuum is a universal constant.
B) electrons have an intrinsic spin.
C) waves have a particle aspect.
D) electrons show wave characteristics.
E) the neutron does not have a charge.

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck
80
An electron that is not localized in space is described by the wave function
= A sin(kx - t). The kinetic energy of the electron is 10 keV. The value of k is approximately
A) 1.6 *10-11 m-1
B) 3.2 * 1013 m-1
C) 1.6 * 1011 m-1
D) 3.2 *1011 m-1
E) 5.1 * 1011 m-1
= A sin(kx - t). The kinetic energy of the electron is 10 keV. The value of k is approximately
A) 1.6 *10-11 m-1
B) 3.2 * 1013 m-1
C) 1.6 * 1011 m-1
D) 3.2 *1011 m-1
E) 5.1 * 1011 m-1

Unlock Deck
Unlock for access to all 153 flashcards in this deck.
Unlock Deck
k this deck


