Exam 14: Wave Particle Duality and Quantum Physics
Exam 1: The Electric Field I: Discrete Charge Distributions87 Questions
Exam 2: The Electric Field II: Continuous Charge Distributions75 Questions
Exam 3: Electric Potential108 Questions
Exam 4: Capacitance73 Questions
Exam 5: Electric Current and Direct-Current Circuits160 Questions
Exam 6: The Magnetic Field71 Questions
Exam 7: Sources of the Magnetic Field115 Questions
Exam 8: Magnetic Induction84 Questions
Exam 9: Alternating-Current Circuits119 Questions
Exam 10: Maxwells Equations and Electromagnetic Waves61 Questions
Exam 11: Properties of Light116 Questions
Exam 12: Optical Images143 Questions
Exam 13: Interference and Diffraction116 Questions
Exam 14: Wave Particle Duality and Quantum Physics153 Questions
Exam 15: Applications of the Schrodinger Equation54 Questions
Exam 16: Atoms128 Questions
Exam 17: Molecules44 Questions
Exam 18: Solids and the Theory of Conduction83 Questions
Exam 19: Relativity83 Questions
Exam 20: Nuclear Physics135 Questions
Exam 21: Elementary Particles and the Beginning of the Universe68 Questions
Select questions type
The wave function for electromagnetic waves is
Free
(Multiple Choice)
4.8/5  (30)
(30)
Correct Answer:
C
An electron in the hydrogen atom (ground-state energy = -13.6 eV) makes a transition from the n = 3 to the n = 1 energy level. Calculate the magnitude of the energy of the photon involved in this process and state whether the photon was absorbed or emitted.
Free
(Multiple Choice)
4.9/5  (32)
(32)
Correct Answer:
A
A classical point particle moves back and forth with constant speed between two walls at x = 0 and x = 4 cm. What is the probability of finding the particle at x = 1 cm?
Free
(Multiple Choice)
4.8/5  (39)
(39)
Correct Answer:
B
Use the following figure for the next two questions. 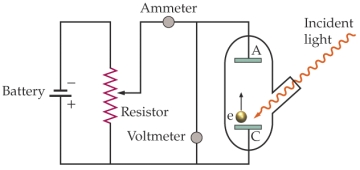 Apparatus for studying the photoelectric effect
-The work function of the material being investigated is 3.8 * 10-19 J. Light of a wavelength 350 nm is incident on the material. What is the lowest voltage needed between the cathode (C) and the anode (A) to stop any electrons ejected from the cathode from reaching the anode?
Apparatus for studying the photoelectric effect
-The work function of the material being investigated is 3.8 * 10-19 J. Light of a wavelength 350 nm is incident on the material. What is the lowest voltage needed between the cathode (C) and the anode (A) to stop any electrons ejected from the cathode from reaching the anode?
(Multiple Choice)
4.9/5  (33)
(33)
Estimate the number of photons emitted by the Sun in a second. The power output from the Sun is 4 *1026 W and assume that the average wavelength of each photon is 550 nm.
(Multiple Choice)
4.9/5  (33)
(33)
A particle is in the ground state of an infinite square-well potential. The probability of finding the particle in x = 0.01L at x = L/4 is
(Multiple Choice)
4.8/5  (36)
(36)
The work function for tungsten is 4.58 eV. What is the kinetic energy of electrons emitted when light of wavelength 400 nm is incident on a tungsten surface? (Planck's constant h = 6.626 * 10-34 J · s = 4.136 * 10-15 eV · s.)
(Multiple Choice)
4.9/5  (42)
(42)
The quantum theory suggests that the stable orbits of electrons around a nucleus correspond to standing waves for the orbit. For a hydrogen atom, the radius of the ground state or the first orbit is the Bohr's radius, 0.0529 nm. What is the wavelength of the ground state of the hydrogen atom?
(Multiple Choice)
4.8/5  (29)
(29)
An electron that is not localized in space is described by the wave function
= A sin(kx - t). The kinetic energy of the electron is 10 keV. The value of k is approximately
(Multiple Choice)
4.8/5  (25)
(25)
When a certain X ray is Compton scattered at right angles to its initial direction, the shift in its wavelength is c = 2.4 pm (1 picometer = 10-12 m). If the wavelength of this X ray is 15.4 pm, the wavelength of the scattered X ray must be closest to
(Multiple Choice)
4.8/5  (39)
(39)
In Compton scattering of X rays by electrons, the maximum shift in the X-ray wavelength occurs when the scattering angle is
(Multiple Choice)
4.8/5  (32)
(32)
A marble of mass 20 g is in a box of length 5.0 cm. The difference between the energies of the two lowest energy states allowed for this marble is approximately
(Multiple Choice)
4.9/5  (29)
(29)
Use the following figure for the next two problems. 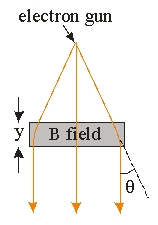 -The electrons coming out of the electron gun in an electron microscope are being accelerated by a potential of 100 kV. The wavelength of the electrons is
-The electrons coming out of the electron gun in an electron microscope are being accelerated by a potential of 100 kV. The wavelength of the electrons is
(Multiple Choice)
4.7/5  (42)
(42)
An electron is in a one-dimensional box of length 0.5 nm. The energy of this electron in its second excited state is
(Multiple Choice)
4.8/5  (38)
(38)
A proton has five times the momentum of an electron. If the electron has a de Broglie wavelength , then the de Broglie wavelength of the proton is
(Multiple Choice)
4.8/5  (28)
(28)
Which of the following experiment(s) illustrates the particle nature of light?
(Multiple Choice)
4.7/5  (29)
(29)
A classical point particle moves back and forth with constant speed between two walls at x = 0 and x = 10 cm. What is the probability of finding the particle between
X = 4.0 cm and x = 5 cm?
(Multiple Choice)
4.9/5  (44)
(44)
Showing 1 - 20 of 153
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)