Deck 17: Molecules
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/44
Play
Full screen (f)
Deck 17: Molecules
1
The bonding of the HF molecule is approximately 43.6% ionic. If its measured electric dipole moment is 6.40 * 10-30 C · m, its equilibrium separation must be
A) 0.0416 nm
B) 0.132 nm
C) 0.0625 nm
D) 0.0917 nm
E) 0.0224 nm
A) 0.0416 nm
B) 0.132 nm
C) 0.0625 nm
D) 0.0917 nm
E) 0.0224 nm
0.0917 nm
2
The _______ bond is the consequence of proton sharing between two atoms.
A) ionic
B) covalent
C) van der Waals
D) hydrogen
E) metallic
A) ionic
B) covalent
C) van der Waals
D) hydrogen
E) metallic
hydrogen
3
The _______ bond is responsible for the bonding of identical or similar atoms.
A) ionic
B) covalent
C) van der Waals
D) hydrogen
E) metallic
A) ionic
B) covalent
C) van der Waals
D) hydrogen
E) metallic
covalent
4
The measured minimum potential energy of a NaCl molecule at stable equilibrium distance of 0.236 nm is -5.7 eV. If the Na+ and Cl- are treated as point charges, the electrostatic potential energy is
A) 2.4 eV
2.4 eV
B) 3.5 eV
3.5 eV
C) 5.4 eV
5.4 eV
D) 6.1 eV
6.1 eV
E) 6.5 eV
6.5 eV
A)
 2.4 eV
2.4 eVB)
 3.5 eV
3.5 eVC)
 5.4 eV
5.4 eVD)
 6.1 eV
6.1 eVE)
 6.5 eV
6.5 eV
Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
5
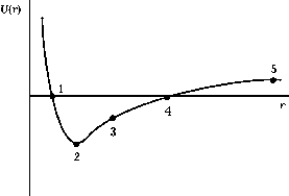 The graph shows the potential energy of the Na+ and Cl- ions as a function of their separation. The point that corresponds to the dissociation energy is
The graph shows the potential energy of the Na+ and Cl- ions as a function of their separation. The point that corresponds to the dissociation energy isA) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
6
The equilibrium separation of the atoms in a given molecule is 0.234 nm and its measured electric dipole moment is 8.76 * 10-30 C · m. What is the percentage of ionic bonding in this molecule?
A) 18%
B) 21%
C) 23%
D) 27%
E) 29%
A) 18%
B) 21%
C) 23%
D) 27%
E) 29%

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
7
When the distance between ions is very small,
A) the electrostatic force becomes very small.
B) the resultant force is the electrostatic force.
C) there is a strong quantum mechanical repulsion.
D) the resultant force is gravitational.
E) None of these is correct.
A) the electrostatic force becomes very small.
B) the resultant force is the electrostatic force.
C) there is a strong quantum mechanical repulsion.
D) the resultant force is gravitational.
E) None of these is correct.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
8
If the equilibrium separation of Na+ and F- ions is 0.193 nm, then their electrostatic potential energy (with U = 0 at infinite separation) must be approximately
A) -1.19 * 10-18 J
B) 1.19 *10-18 J
C) 7.42 J
D) -7.42 J
E) 4.64 * 1019 J
A) -1.19 * 10-18 J
B) 1.19 *10-18 J
C) 7.42 J
D) -7.42 J
E) 4.64 * 1019 J

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
9
The bonding of the HF molecule is approximately 43.6% ionic. If its equilibrium separation is 0.917 nm, then its electric dipole moment must be approximately
A) 3.20 * 10-30 C · m
B) 6.40 * 10-30 C · m
C) 8.76 * 10-30 C · m
D) 11.4 * 10-30 C · m
E) 13.2 * 10-30 C · m
A) 3.20 * 10-30 C · m
B) 6.40 * 10-30 C · m
C) 8.76 * 10-30 C · m
D) 11.4 * 10-30 C · m
E) 13.2 * 10-30 C · m

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
10
Which one of the following statements is true of the van der Waals bond?
A) Any two separated molecules will be attracted to one another by electrostatic forces.
B) The van der Waals bonds due to electrostatic forces are weaker than the ionic and covalent bonds.
C) At high temperatures, the van der Waals forces are not strong enough to overcome the ordinary thermal agitation of atoms or molecules.
D) At sufficiently low temperatures, the van der Waals forces will cause virtually all substances to condense into a liquid and then a solid form.
E) All of these are correct.
A) Any two separated molecules will be attracted to one another by electrostatic forces.
B) The van der Waals bonds due to electrostatic forces are weaker than the ionic and covalent bonds.
C) At high temperatures, the van der Waals forces are not strong enough to overcome the ordinary thermal agitation of atoms or molecules.
D) At sufficiently low temperatures, the van der Waals forces will cause virtually all substances to condense into a liquid and then a solid form.
E) All of these are correct.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
11
The _______ bond is based on the sharing of each valence electron by many atoms.
A) ionic
B) covalent
C) van der Waals
D) hydrogen
E) metallic
A) ionic
B) covalent
C) van der Waals
D) hydrogen
E) metallic

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
12
If the electrostatic potential energy of Na+ and F- ions at their equilibrium separation (with U = 0 at infinite separation) is -1.19 * 10-18 J, then their equilibrium separation must be approximately
A) 2.14 nm
B) 0.193 nm
C) 0.876 nm
D) 1.32 nm
E) 0.374 nm
A) 2.14 nm
B) 0.193 nm
C) 0.876 nm
D) 1.32 nm
E) 0.374 nm

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
13
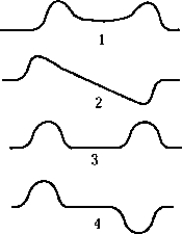 The symmetric wave function for two identical, widely separated, finite square wells is best represented by which of the graphs?
The symmetric wave function for two identical, widely separated, finite square wells is best represented by which of the graphs?A) 1
B) 2
C) 3
D) 4
E) All of these are correct.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
14
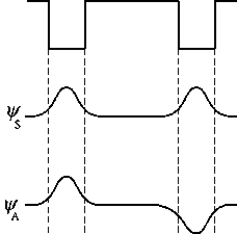 The figure represents two square wells that are far apart and the symmetric ( S) and antisymmetric ( A) electron wave functions. Which of the following statements correctly describes the probability distributions and the energies of the wave functions?
The figure represents two square wells that are far apart and the symmetric ( S) and antisymmetric ( A) electron wave functions. Which of the following statements correctly describes the probability distributions and the energies of the wave functions?A) The energies are the same for these two wave functions but the probability distributions are different.
B) Neither the energies nor the probability distributions are the same for these two wave functions.
C) The probability distributions and energies are the same for these two wave functions.
D) The probability distributions are the same for these two wave functions but the energies are different.
E) None of these is correct.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
15
The dissociation energy of an ionic compound is the negative of the potential energy of the positive-negative ion system when the ions are
A) touching.
B) infinitely far apart.
C) still atoms and have not yet been ionized.
D) at the equilibrium separation.
E) completely dissolved in a solvent.
A) touching.
B) infinitely far apart.
C) still atoms and have not yet been ionized.
D) at the equilibrium separation.
E) completely dissolved in a solvent.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
16
The _______ bond is responsible for the bonding of most salts.
A) ionic
B) covalent
C) van der Waals
D) hydrogen
E) metallic
A) ionic
B) covalent
C) van der Waals
D) hydrogen
E) metallic

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
17
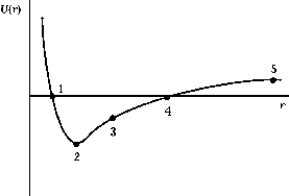 The graph shows the potential energy of the Na+ and Cl- ions as a function of their separation. The point that corresponds to the equilibrium separation is
The graph shows the potential energy of the Na+ and Cl- ions as a function of their separation. The point that corresponds to the equilibrium separation isA) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
18
The energy required to ionize sodium is 5.14 eV. If the energy needed to form Na+ and F- ions from neutral sodium and fluorine atoms is 1.69 eV, the electron affinity of fluorine is
A) 3.45 eV
B) 5.14 eV
C) 1.69 eV
D) 6.83 eV
E) 2.79 eV
A) 3.45 eV
B) 5.14 eV
C) 1.69 eV
D) 6.83 eV
E) 2.79 eV

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
19
When a NaCl molecule is formed from neutral Na and Cl atoms,
A) a net input of energy is required.
B) there is no net output of energy.
C) a net output of energy is obtained.
D) the molecule forms a covalent bond.
E) None of the above is correct.
A) a net input of energy is required.
B) there is no net output of energy.
C) a net output of energy is obtained.
D) the molecule forms a covalent bond.
E) None of the above is correct.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
20
The energy released by an atom's acquisition of one electron is called its
A) molecular orbital energy.
B) ionic bond.
C) exclusion-principle repulsion.
D) acquisition energy.
E) electron affinity.
A) molecular orbital energy.
B) ionic bond.
C) exclusion-principle repulsion.
D) acquisition energy.
E) electron affinity.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
21
The reduced mass of a two-body system in which the masses of the bodies are 2 u and
5 u is
A) 3.50 u
B) 1.40 u
C) 1.73 u
D) 0.71 u
E) 0.29 u
5 u is
A) 3.50 u
B) 1.40 u
C) 1.73 u
D) 0.71 u
E) 0.29 u

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
22
Transitions between vibrational states of different electronic states result in the emission of photons in or near the _______ portion of the electromagnetic spectrum.
A) microwave
B) visible
C) far infrared
D) X-ray
E) ultraviolet
A) microwave
B) visible
C) far infrared
D) X-ray
E) ultraviolet

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
23
The magnetron of a microwave oven generates a frequency of 2.45 GHz. This frequency corresponds to the first vibrational level, 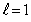 , of a water molecule. Assuming that the energy is given by
, of a water molecule. Assuming that the energy is given by 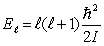 , the moment of inertia of the water molecule is
, the moment of inertia of the water molecule is
A) 7.81 * 10-46 kg.m2
B) 3.23 * 10-45 kg.m2
C) 1.37 *10-45 kg.m2
D) 3.44 * 10-45 kg.m2
E) 6.85 * 10-45 kg.m2
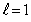 , of a water molecule. Assuming that the energy is given by
, of a water molecule. Assuming that the energy is given by 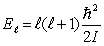 , the moment of inertia of the water molecule is
, the moment of inertia of the water molecule isA) 7.81 * 10-46 kg.m2
B) 3.23 * 10-45 kg.m2
C) 1.37 *10-45 kg.m2
D) 3.44 * 10-45 kg.m2
E) 6.85 * 10-45 kg.m2

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
24
The reduced mass of a two-body system is 2.1 u. If one of the masses is 3 u, then what is the mass of the second body?
A) 5 u
B) 7 u
C) 9 u
D) 6 u
E) 4 u
A) 5 u
B) 7 u
C) 9 u
D) 6 u
E) 4 u

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
25
From the frequency of vibration the effective force constant of the HCl molecule is found to be 840 N/m. In order to appreciate the stiffness of the binding force, or imaginary "spring," between the H and Cl, calculate how far a spring of the same stiffness would extend if a 10 kg weight were hung from it.
A) 0.12 cm
B) 8.3 m
C) 12 mm
D) 8.3 mm
E) 12 cm
A) 0.12 cm
B) 8.3 m
C) 12 mm
D) 8.3 mm
E) 12 cm

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
26
The energy of a diatomic molecule can best be understood in terms of
A) electronic motion.
B) vibrational motion.
C) rotational motion.
D) electronic and vibrational motions.
E) electronic, vibrational, and rotational motions.
A) electronic motion.
B) vibrational motion.
C) rotational motion.
D) electronic and vibrational motions.
E) electronic, vibrational, and rotational motions.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
27
The first two non-zero rotational energies of a N2 molecule are 0.493 meV and 1.48 meV. The two nitrogen atoms are separated by
A) 0.110 nm
B) 0.192 nm
C) 0.214 nm
D) 0.096 nm
E) 0.089 nm
A) 0.110 nm
B) 0.192 nm
C) 0.214 nm
D) 0.096 nm
E) 0.089 nm

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
28
If the H2 molecule has a rotational energy of E0r = 0.040 MeV, what is the average distance between the two atoms?
A) 1.0 * 10-13 m
B) 3.2 * 10-11 m
C) 1.0 * 10-10 m
D) 1.0 * 10-20 m
E) 1.4 * 10-10 m
A) 1.0 * 10-13 m
B) 3.2 * 10-11 m
C) 1.0 * 10-10 m
D) 1.0 * 10-20 m
E) 1.4 * 10-10 m

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
29
The separation distance of the atoms in N2 (mass = 28 u) is 0.0773 nm. The characteristic rotational energy E0r for the N2 molecule is approximately
A) 3.27* 10-4 eV
B) 2.48 * 10-4 eV
C) 4.96 * 10-4 eV
D) 1.24 * 10-4 eV
E) 1.58 *10-4 eV
A) 3.27* 10-4 eV
B) 2.48 * 10-4 eV
C) 4.96 * 10-4 eV
D) 1.24 * 10-4 eV
E) 1.58 *10-4 eV

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
30
The mass of the cesium atom is 133 u and that of the chlorine atom is 35.5 u. The reduced mass of CsCl is approximately
A) 28.0 u
B) 169 u
C) 97.5 u
D) 84.3 u
E) 63.7 u
A) 28.0 u
B) 169 u
C) 97.5 u
D) 84.3 u
E) 63.7 u

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
31
The two oxygen atoms in the O2 molecule are separated by 0.12 nm. What is the wavelength of the photon emitted when the O2 goes from 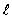 = 2 to
= 2 to 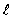 = 1 rotational states?
= 1 rotational states?
A) 38 mm
B) 34 mm
C) 1.1 mm
D) 1.7 mm
E) 6.9 mm
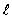 = 2 to
= 2 to 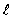 = 1 rotational states?
= 1 rotational states?A) 38 mm
B) 34 mm
C) 1.1 mm
D) 1.7 mm
E) 6.9 mm

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
32
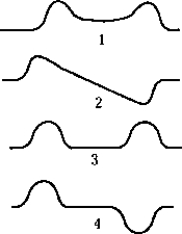 The antisymmetric wave function for two identical, closely spaced, finite square wells is best represented by which of the graphs?
The antisymmetric wave function for two identical, closely spaced, finite square wells is best represented by which of the graphs?A) 1
B) 2
C) 3
D) 4
E) All of these are correct.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following statements about covalent bonding is true?
A) In molecules with two or more bonds, the angles between the bonds are fixed.
B) Each covalent bond involves the sharing of one electron each from two atoms.
C) The two electrons forming a covalent bond must have opposite spins.
D) An atom can form more than one covalent bond.
E) All of the above statements are correct.
A) In molecules with two or more bonds, the angles between the bonds are fixed.
B) Each covalent bond involves the sharing of one electron each from two atoms.
C) The two electrons forming a covalent bond must have opposite spins.
D) An atom can form more than one covalent bond.
E) All of the above statements are correct.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
34
The energies due to the electronic excitations of a molecule are of the order of 1 eV, whereas
A) the energies of vibration and rotation are much larger than this.
B) the energy of vibration is much larger and the energy of rotation much smaller than this.
C) the energies of vibration and rotation are of this same order of magnitude.
D) the energies of vibration and rotation are found to be both larger and smaller than 1 eV.
E) the energies of vibration and rotation are much smaller than this.
A) the energies of vibration and rotation are much larger than this.
B) the energy of vibration is much larger and the energy of rotation much smaller than this.
C) the energies of vibration and rotation are of this same order of magnitude.
D) the energies of vibration and rotation are found to be both larger and smaller than 1 eV.
E) the energies of vibration and rotation are much smaller than this.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
35
The two oxygen atoms in the O2 molecule are separated by 0.12 nm. What are the values of the two lowest nonzero rotational energies of O2?
A) 9.0 * 10-5 eV and 1.8 * 10-4 eV
B) 3.6 * 10-4 eV and 11 *10-4 eV
C) 3.6 *10-4 eV and 7.2 *10-4 eV
D) 1.8 * 10-4 eV and 11*10-4 eV
E) 3.6 * 10-4 eV and 22 *10-4 eV
A) 9.0 * 10-5 eV and 1.8 * 10-4 eV
B) 3.6 * 10-4 eV and 11 *10-4 eV
C) 3.6 *10-4 eV and 7.2 *10-4 eV
D) 1.8 * 10-4 eV and 11*10-4 eV
E) 3.6 * 10-4 eV and 22 *10-4 eV

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
36
When examined with a grating spectrograph, the discharge from helium gas has bright radiation at a relatively few discrete wavelengths; the discharge from nitrogen gas has radiation in bands with a very large number of discrete wavelengths. What is the reason for this difference in the spectra of these two gases?
A) The atoms of the helium gas have two electrons whereas the atoms of the nitrogen gas have many electrons; therefore, the nitrogen gas gives a more complicated spectrum.
B) The nitrogen gas has molecules that have rotational and vibrational energies that produce bands of closely spaced wavelengths in the spectrum.
C) The nitrogen gas discharge is hotter, which "smears out" the wavelengths of the radiation according to Planck's law for blackbody radiation.
D) The levels of the helium atoms can be obtained from the Bohr theory of atoms, which gives a few relatively discrete allowed energies, whereas nitrogen must be described by the band theory, which gives continuous bands of allowed energies.
E) None of these is correct.
A) The atoms of the helium gas have two electrons whereas the atoms of the nitrogen gas have many electrons; therefore, the nitrogen gas gives a more complicated spectrum.
B) The nitrogen gas has molecules that have rotational and vibrational energies that produce bands of closely spaced wavelengths in the spectrum.
C) The nitrogen gas discharge is hotter, which "smears out" the wavelengths of the radiation according to Planck's law for blackbody radiation.
D) The levels of the helium atoms can be obtained from the Bohr theory of atoms, which gives a few relatively discrete allowed energies, whereas nitrogen must be described by the band theory, which gives continuous bands of allowed energies.
E) None of these is correct.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
37
The effective force constant of the CO molecule is 1.86 kN/m. With 12 u for the mass of the carbon atom and 16 u for the mass of the oxygen atom, the frequency of vibration of the CO molecule is
A) 14.2 *1013 Hz
B) 2.47 * 1013 Hz
C) 6.42 * 1013 Hz
D) 9.76 * 1013 Hz
E) 5.93 * 1013 Hz
A) 14.2 *1013 Hz
B) 2.47 * 1013 Hz
C) 6.42 * 1013 Hz
D) 9.76 * 1013 Hz
E) 5.93 * 1013 Hz

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
38
Transitions within a given set of rotational energy levels yield photons in the _________ portion of the electromagnetic spectrum.
A) microwave
B) visible
C) far infrared
D) X-ray
E) ultraviolet
A) microwave
B) visible
C) far infrared
D) X-ray
E) ultraviolet

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
39
Gases such as CO2 and methane are considered greenhouse gases. This is because
A) they are the most common in the atmosphere allowing them to trap more heat than the other gases.
B) they form covalent bonds and hence can easily act as a shield for heat.
C) the vibrational modes of the molecules are in the infrared region which imply that they readily absorb radiant heat thus preventing it from escaping into space.
D) the gases are formed by human activity resulting in offsetting the natural balance in the gas concentrations.
E) None of the above statements is correct.
A) they are the most common in the atmosphere allowing them to trap more heat than the other gases.
B) they form covalent bonds and hence can easily act as a shield for heat.
C) the vibrational modes of the molecules are in the infrared region which imply that they readily absorb radiant heat thus preventing it from escaping into space.
D) the gases are formed by human activity resulting in offsetting the natural balance in the gas concentrations.
E) None of the above statements is correct.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
40
The reduced mass of a system of two particles whose masses are m1 and m2 is
A) µ = (m1 + m2)/m1 · m2
B) µ = (m1 + m2)/m1
C) µ = m1/(m1 + m2)
D) µ = m1 · m2/m1
E) µ = m1 · m2/(m1 + m2)
A) µ = (m1 + m2)/m1 · m2
B) µ = (m1 + m2)/m1
C) µ = m1/(m1 + m2)
D) µ = m1 · m2/m1
E) µ = m1 · m2/(m1 + m2)

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
41
In the absorption spectrum of the diatomic molecule HCl the central frequency is at
8)67 * 1013 Hz, while the absorption peaks on either side are separated by 5.9 *1011 Hz. What is the average moment of inertia of a rotating HCl molecule?
A) 1.4 *10-47 kg.m2
B) 7.1 * 10-48 kg.m2
C) 1.1 * 10-45 kg.m2
D) 2.8 *10-47 kg.m2
E) None of these is correct.
8)67 * 1013 Hz, while the absorption peaks on either side are separated by 5.9 *1011 Hz. What is the average moment of inertia of a rotating HCl molecule?
A) 1.4 *10-47 kg.m2
B) 7.1 * 10-48 kg.m2
C) 1.1 * 10-45 kg.m2
D) 2.8 *10-47 kg.m2
E) None of these is correct.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
42
The characteristic energy of rotation E0r for the O2 molecule is 1.78 *10-4 eV. Calculate the frequency of the photon absorbed for the excitation from the l = 3 to l = 4 rotational level of this diatomic molecule.
A) 4.3 * 1010 Hz
B) 3.0 *1011 Hz
C) 1.9 *1011 Hz
D) 3.4 * 1011 Hz
E) 5.6* 1011 Hz
A) 4.3 * 1010 Hz
B) 3.0 *1011 Hz
C) 1.9 *1011 Hz
D) 3.4 * 1011 Hz
E) 5.6* 1011 Hz

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
43
In the absorption spectrum of the diatomic molecule HCl the central frequency is at
8)67 *1013 Hz, while the absorption peaks on either side are separated by 5.9 *1011 Hz. What is the energy of the lowest vibrational state?
A) 0.18 eV
B) 0.27 eV
C) 0.36 eV
D) 2.4 meV
E) 1.2 meV
8)67 *1013 Hz, while the absorption peaks on either side are separated by 5.9 *1011 Hz. What is the energy of the lowest vibrational state?
A) 0.18 eV
B) 0.27 eV
C) 0.36 eV
D) 2.4 meV
E) 1.2 meV

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
44
In the absorption spectrum of the diatomic molecule HCl the central frequency is at
8)67 * 1013 Hz, while the absorption peaks on either side are separated by 5.9 *1011 Hz. What is the difference in energy between neighboring vibrational levels?
A) 0.18 eV
B) 0.27 eV
C) 0.36 eV
D) 2.4 meV
E) 1.2 meV
8)67 * 1013 Hz, while the absorption peaks on either side are separated by 5.9 *1011 Hz. What is the difference in energy between neighboring vibrational levels?
A) 0.18 eV
B) 0.27 eV
C) 0.36 eV
D) 2.4 meV
E) 1.2 meV

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck


