Exam 17: Molecules
Exam 1: The Electric Field I: Discrete Charge Distributions87 Questions
Exam 2: The Electric Field II: Continuous Charge Distributions75 Questions
Exam 3: Electric Potential108 Questions
Exam 4: Capacitance73 Questions
Exam 5: Electric Current and Direct-Current Circuits160 Questions
Exam 6: The Magnetic Field71 Questions
Exam 7: Sources of the Magnetic Field115 Questions
Exam 8: Magnetic Induction84 Questions
Exam 9: Alternating-Current Circuits119 Questions
Exam 10: Maxwells Equations and Electromagnetic Waves61 Questions
Exam 11: Properties of Light116 Questions
Exam 12: Optical Images143 Questions
Exam 13: Interference and Diffraction116 Questions
Exam 14: Wave Particle Duality and Quantum Physics153 Questions
Exam 15: Applications of the Schrodinger Equation54 Questions
Exam 16: Atoms128 Questions
Exam 17: Molecules44 Questions
Exam 18: Solids and the Theory of Conduction83 Questions
Exam 19: Relativity83 Questions
Exam 20: Nuclear Physics135 Questions
Exam 21: Elementary Particles and the Beginning of the Universe68 Questions
Select questions type
When the distance between ions is very small,
Free
(Multiple Choice)
4.9/5  (35)
(35)
Correct Answer:
C
When a NaCl molecule is formed from neutral Na and Cl atoms,
Free
(Multiple Choice)
4.8/5  (22)
(22)
Correct Answer:
C
The _______ bond is based on the sharing of each valence electron by many atoms.
Free
(Multiple Choice)
4.7/5  (30)
(30)
Correct Answer:
E
The bonding of the HF molecule is approximately 43.6% ionic. If its measured electric dipole moment is 6.40 * 10-30 C · m, its equilibrium separation must be
(Multiple Choice)
4.7/5  (33)
(33)
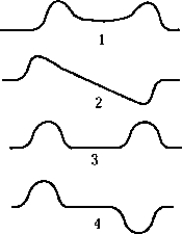 The symmetric wave function for two identical, widely separated, finite square wells is best represented by which of the graphs?
The symmetric wave function for two identical, widely separated, finite square wells is best represented by which of the graphs?
(Multiple Choice)
4.9/5  (43)
(43)
The energy of a diatomic molecule can best be understood in terms of
(Multiple Choice)
4.9/5  (41)
(41)
In the absorption spectrum of the diatomic molecule HCl the central frequency is at
8)67 *1013 Hz, while the absorption peaks on either side are separated by 5.9 *1011 Hz. What is the energy of the lowest vibrational state?
(Multiple Choice)
4.8/5  (31)
(31)
The effective force constant of the CO molecule is 1.86 kN/m. With 12 u for the mass of the carbon atom and 16 u for the mass of the oxygen atom, the frequency of vibration of the CO molecule is
(Multiple Choice)
4.8/5  (32)
(32)
The characteristic energy of rotation E0r for the O2 molecule is 1.78 *10-4 eV. Calculate the frequency of the photon absorbed for the excitation from the l = 3 to l = 4 rotational level of this diatomic molecule.
(Multiple Choice)
4.8/5  (28)
(28)
The two oxygen atoms in the O2 molecule are separated by 0.12 nm. What is the wavelength of the photon emitted when the O2 goes from 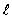 = 2 to
= 2 to  = 1 rotational states?
= 1 rotational states?
(Multiple Choice)
4.9/5  (33)
(33)
The equilibrium separation of the atoms in a given molecule is 0.234 nm and its measured electric dipole moment is 8.76 * 10-30 C · m. What is the percentage of ionic bonding in this molecule?
(Multiple Choice)
4.9/5  (33)
(33)
The separation distance of the atoms in N2 (mass = 28 u) is 0.0773 nm. The characteristic rotational energy E0r for the N2 molecule is approximately
(Multiple Choice)
4.8/5  (39)
(39)
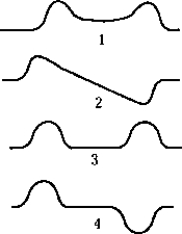 The antisymmetric wave function for two identical, closely spaced, finite square wells is best represented by which of the graphs?
The antisymmetric wave function for two identical, closely spaced, finite square wells is best represented by which of the graphs?
(Multiple Choice)
4.8/5  (27)
(27)
Gases such as CO2 and methane are considered greenhouse gases. This is because
(Multiple Choice)
4.8/5  (33)
(33)
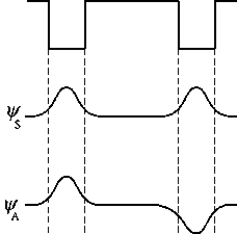 The figure represents two square wells that are far apart and the symmetric ( S) and antisymmetric ( A) electron wave functions. Which of the following statements correctly describes the probability distributions and the energies of the wave functions?
The figure represents two square wells that are far apart and the symmetric ( S) and antisymmetric ( A) electron wave functions. Which of the following statements correctly describes the probability distributions and the energies of the wave functions?
(Multiple Choice)
4.9/5  (32)
(32)
The two oxygen atoms in the O2 molecule are separated by 0.12 nm. What are the values of the two lowest nonzero rotational energies of O2?
(Multiple Choice)
4.9/5  (36)
(36)
In the absorption spectrum of the diatomic molecule HCl the central frequency is at
8)67 * 1013 Hz, while the absorption peaks on either side are separated by 5.9 *1011 Hz. What is the average moment of inertia of a rotating HCl molecule?
(Multiple Choice)
4.9/5  (30)
(30)
Which one of the following statements is true of the van der Waals bond?
(Multiple Choice)
4.8/5  (35)
(35)
The first two non-zero rotational energies of a N2 molecule are 0.493 meV and 1.48 meV. The two nitrogen atoms are separated by
(Multiple Choice)
4.8/5  (33)
(33)
The dissociation energy of an ionic compound is the negative of the potential energy of the positive-negative ion system when the ions are
(Multiple Choice)
4.7/5  (36)
(36)
Showing 1 - 20 of 44
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)