Deck 19: The Second Law of Thermodynamics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/61
Play
Full screen (f)
Deck 19: The Second Law of Thermodynamics
1
Use the following diagram to answer the next problem. 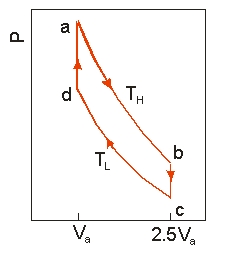
An ideal heat engine uses 0.01 mol of gas and operates between a hot reservoir at TH = 400 K and cold reservoir at TL = 300 K, in a cycle from a b c d a. From a b the gas undergoes an isothermal expansion, changing its volume from Va to 2.5Va. From b c, the pressure is reduced at a constant volume. From c d, the gas undergoes an isothermal compression, and from d a, the pressure is increased at a constant volume until the gas is back at the original condition at a.
-How much work is obtained from the engine in each cycle?
A) 22.9 J
B) 30.5 J
C) 7.62 J
D) 8.31 J
E) 0.917 J

An ideal heat engine uses 0.01 mol of gas and operates between a hot reservoir at TH = 400 K and cold reservoir at TL = 300 K, in a cycle from a b c d a. From a b the gas undergoes an isothermal expansion, changing its volume from Va to 2.5Va. From b c, the pressure is reduced at a constant volume. From c d, the gas undergoes an isothermal compression, and from d a, the pressure is increased at a constant volume until the gas is back at the original condition at a.
-How much work is obtained from the engine in each cycle?
A) 22.9 J
B) 30.5 J
C) 7.62 J
D) 8.31 J
E) 0.917 J
7.62 J
2
An engine operating in a cycle would violate the second law of thermodynamics if it
A) changed all the heat from a source to mechanical work.
B) changed all of its mechanical work to heat.
C) were irreversible.
D) operated between two isotherms and two adiabats.
E) were less efficient than a Carnot engine.
A) changed all the heat from a source to mechanical work.
B) changed all of its mechanical work to heat.
C) were irreversible.
D) operated between two isotherms and two adiabats.
E) were less efficient than a Carnot engine.
changed all the heat from a source to mechanical work.
3
A heat engine with an output of 300 W has an efficiency of 25% and works at 10 cycles/s. How much heat is absorbed (Qh) and how much rejected (Qc) in each cycle?
A) Qh = 150 J, Qc = 120 J
B) Qh = 1500 J, Qc = 1200 J
C) Qh = 40 J, Qc = 10 J
D) Qh = 120 J, Qc = 90 J
E) Qh = 1200 J, Qc = 900 J
A) Qh = 150 J, Qc = 120 J
B) Qh = 1500 J, Qc = 1200 J
C) Qh = 40 J, Qc = 10 J
D) Qh = 120 J, Qc = 90 J
E) Qh = 1200 J, Qc = 900 J
Qh = 120 J, Qc = 90 J
4
A heat engine operating between the temperatures T1 and T2 takes in Q1 calories at temperature T1 and gives up Q2 calories at temperature T2. The efficiency of this heat engine is
A) (Q1 - Q2)/Q2
B) (Q1 - Q2)/Q1
C) (T2 - T1)/T2
D) Q2/(Q1 - Q2)
E) T1/(T1 - T2)
A) (Q1 - Q2)/Q2
B) (Q1 - Q2)/Q1
C) (T2 - T1)/T2
D) Q2/(Q1 - Q2)
E) T1/(T1 - T2)

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
5
A refrigerator has a coefficient of performance 5.0. How much heat is exhausted to the hot reservoir when 200 kJ of heat are removed from the cold reservoir?
A) 50 kJ
B) 150 kJ
C) 200 kJ
D) 240 kJ
E) Not enough information is given to answer this question.
A) 50 kJ
B) 150 kJ
C) 200 kJ
D) 240 kJ
E) Not enough information is given to answer this question.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
6
A substance undergoes a series of reversible processes that bring it back to its initial state. In this cycle, heat Qx is absorbed by the substance and heat Qy is rejected. The net amount of work performed by the substance is
A) Qy - Qx
B) Qx - Qy
C) (Qy - Qx)/Qy
D) (Qx - Qy)/Qx
E) (Qx - Qy)/Qy
A) Qy - Qx
B) Qx - Qy
C) (Qy - Qx)/Qy
D) (Qx - Qy)/Qx
E) (Qx - Qy)/Qy

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
7
Two refrigerators, one with a COP of 4.0 and another with a COP of 5.0, both extract 400 kJ of heat from the cold reservoir (food). Calculate the difference in energy they exhaust to the hot reservoir and hence the room.
A) 320 kJ
B) 80 kJ
C) 125 kJ
D) 100 kJ
E) 20 kJ
A) 320 kJ
B) 80 kJ
C) 125 kJ
D) 100 kJ
E) 20 kJ

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
8
For which of the following is COP the abbreviation?
A) Clausius or Planck
B) cycle of pressure
C) coefficients of pressure
D) Carnot ordered performance
E) coefficient of performance
A) Clausius or Planck
B) cycle of pressure
C) coefficients of pressure
D) Carnot ordered performance
E) coefficient of performance

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
9
A heat engine absorbs 70 kcal of heat from a hot reservoir and exhausts 50 kcal to a cold reservoir each cycle. Its efficiency is
A) 20%
B) 24%
C) 29%
D) 33%
E) 37%
A) 20%
B) 24%
C) 29%
D) 33%
E) 37%

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
10
A refrigerator extracts heat Q from a cold reservoir. The heat exhausted to a hot reservoir
A) is Q.
B) must be greater than Q.
C) must be less than Q.
D) could be greater than Q.
E) is zero.
A) is Q.
B) must be greater than Q.
C) must be less than Q.
D) could be greater than Q.
E) is zero.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
11
A refrigerator with a coefficient of performance 5.0 removes 25 kJ of heat from a cold reservoir. If this refrigerator is reversible and is run backward as a heat engine, what would be the efficiency of the heat engine?
A) 50%
B) 80%
C) 83%
D) 17%
E) 20%
A) 50%
B) 80%
C) 83%
D) 17%
E) 20%

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
12
Use the following diagram to answer the next problem. 
An ideal heat engine uses 0.01 mol of gas and operates between a hot reservoir at TH = 400 K and cold reservoir at TL = 300 K, in a cycle from a b c d a. From a b the gas undergoes an isothermal expansion, changing its volume from Va to 2.5Va. From b c, the pressure is reduced at a constant volume. From c d, the gas undergoes an isothermal compression, and from d a, the pressure is increased at a constant volume until the gas is back at the original condition at a.
-If the engine operates at 50 cycles per second, the power output is
A) 381 W
B) 45.8 W
C) 1145 W
D) 415 W
E) 1525 W

An ideal heat engine uses 0.01 mol of gas and operates between a hot reservoir at TH = 400 K and cold reservoir at TL = 300 K, in a cycle from a b c d a. From a b the gas undergoes an isothermal expansion, changing its volume from Va to 2.5Va. From b c, the pressure is reduced at a constant volume. From c d, the gas undergoes an isothermal compression, and from d a, the pressure is increased at a constant volume until the gas is back at the original condition at a.
-If the engine operates at 50 cycles per second, the power output is
A) 381 W
B) 45.8 W
C) 1145 W
D) 415 W
E) 1525 W

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
13
A heat engine absorbs 150 J of heat from a hot reservoir and rejects 90 J to a cold reservoir. What is the efficiency of this engine?
A) 20%
B) 40%
C) 60%
D) 67%
E) 90%
A) 20%
B) 40%
C) 60%
D) 67%
E) 90%

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
14
Use the following diagram to answer the next problem. 
An ideal heat engine uses 0.01 mol of gas and operates between a hot reservoir at TH = 400 K and cold reservoir at TL = 300 K, in a cycle from a b c d a. From a b the gas undergoes an isothermal expansion, changing its volume from Va to 2.5Va. From b c, the pressure is reduced at a constant volume. From c d, the gas undergoes an isothermal compression, and from d a, the pressure is increased at a constant volume until the gas is back at the original condition at a.
-How much heat is absorbed in going from a b?
A) 30.5 J
B) 7.62 J
C) 22.9 J
D) 8.31 J
E) 0.917 J

An ideal heat engine uses 0.01 mol of gas and operates between a hot reservoir at TH = 400 K and cold reservoir at TL = 300 K, in a cycle from a b c d a. From a b the gas undergoes an isothermal expansion, changing its volume from Va to 2.5Va. From b c, the pressure is reduced at a constant volume. From c d, the gas undergoes an isothermal compression, and from d a, the pressure is increased at a constant volume until the gas is back at the original condition at a.
-How much heat is absorbed in going from a b?
A) 30.5 J
B) 7.62 J
C) 22.9 J
D) 8.31 J
E) 0.917 J

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
15
A heat engine exhausts heat Q to a cold reservoir. The amount of work done by the engine
A) is Q.
B) must be greater than Q.
C) must be less than Q.
D) could be greater than Q.
E) is zero.
A) is Q.
B) must be greater than Q.
C) must be less than Q.
D) could be greater than Q.
E) is zero.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
16
A heat engine absorbs heat Q from a hot reservoir. The amount of work done by the engine
A) is Q.
B) must be greater than Q.
C) must be less than Q.
D) could be greater than Q.
E) is zero.
A) is Q.
B) must be greater than Q.
C) must be less than Q.
D) could be greater than Q.
E) is zero.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
17
A heat engine absorbs 64 kcal of heat from a hot reservoir and exhausts 42 kcal to a cold reservoir each cycle. Its efficiency is
A) 30%
B) 34%
C) 38%
D) 40%
E) 42%
A) 30%
B) 34%
C) 38%
D) 40%
E) 42%

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
18
If you run a refrigerator in a closed room with the door to the refrigerator open, the temperature of the room
A) increases.
B) remains the same.
C) decreases.
D) Any of these can happen depending on how efficient the refrigerator is.
E) Any of these can happen depending on the relative sizes of the room and the refrigerator.
A) increases.
B) remains the same.
C) decreases.
D) Any of these can happen depending on how efficient the refrigerator is.
E) Any of these can happen depending on the relative sizes of the room and the refrigerator.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
19
A refrigerator extracts 25 kJ from a cold reservoir and rejects 35 kJ to a hot reservoir. What is the coefficient of performance of this refrigerator?
A) 2.5
B) 3.5
C) 1.4
D) 5.0
E) 4.0
A) 2.5
B) 3.5
C) 1.4
D) 5.0
E) 4.0

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
20
The diagram below is a schematic of a heat engine. The three quantities, QH, QL, and W are represented, respectively, by 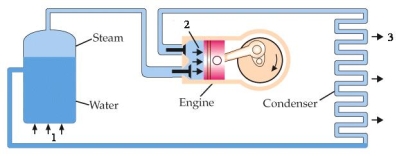
A) 1, 2, 3
B) 1, 3, 2
C) 2, 3, 1
D) 3, 1, 2
E) 3, 2, 1

A) 1, 2, 3
B) 1, 3, 2
C) 2, 3, 1
D) 3, 1, 2
E) 3, 2, 1

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
21
The efficiency of a Carnot engine operating between 300º C and 250ºC is about
A) 83%
B) 17%
C) 57%
D) 43%
E) 9%
A) 83%
B) 17%
C) 57%
D) 43%
E) 9%

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
22
The maximum theoretical thermal efficiency of a steam engine that is supplied steam at a temperature of 600ºC and exhausts it at a temperature of 200ºC is
A) 33.3%
B) 45.8%
C) 66.7%
D) 77.1%
E) 84.6%
A) 33.3%
B) 45.8%
C) 66.7%
D) 77.1%
E) 84.6%

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
23
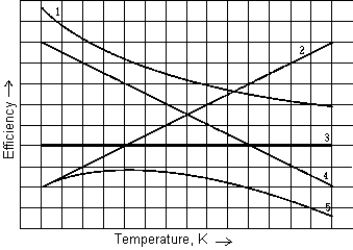 You can vary the efficiency of a Carnot engine by varying the temperature of the cold reservoir while maintaining the hot reservoir at constant temperature. The curve that best represents the efficiency of such an engine as a function of the temperature of the cold reservoir is
You can vary the efficiency of a Carnot engine by varying the temperature of the cold reservoir while maintaining the hot reservoir at constant temperature. The curve that best represents the efficiency of such an engine as a function of the temperature of the cold reservoir isA) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
24
The construction of a heat engine operating between temperatures T1 and T2, and having an efficiency greater than that of a Carnot engine operating between the same two temperatures, would constitute a violation of
A) the law of the conservation of energy.
B) the first law of thermodynamics.
C) the second law of thermodynamics.
D) Boyle's law.
E) None of these is correct.
A) the law of the conservation of energy.
B) the first law of thermodynamics.
C) the second law of thermodynamics.
D) Boyle's law.
E) None of these is correct.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
25
The Carnot cycle consists of
A) a quasistatic isothermal absorption of heat from a hot reservoir.
B) a quasistatic adiabatic expansion to a lower temperature.
C) a quasistatic isothermal exhaustion of heat to a cold reservoir.
D) a quasistatic adiabatic compression to the initial state of the system.
E) All of these are correct.
A) a quasistatic isothermal absorption of heat from a hot reservoir.
B) a quasistatic adiabatic expansion to a lower temperature.
C) a quasistatic isothermal exhaustion of heat to a cold reservoir.
D) a quasistatic adiabatic compression to the initial state of the system.
E) All of these are correct.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
26
A Carnot engine operating between reservoir temperatures of 340º C and 40ºC has an efficiency of
A) 37.5%
B) 49.0%
C) 60.0%
D) 62.5%
E) 88.2%
A) 37.5%
B) 49.0%
C) 60.0%
D) 62.5%
E) 88.2%

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
27
We wish to increase the efficiency of an ideal heat engine from 25% to 35%. If the initial temperature of the hot reservoir is 650 C, to what temperature would this need to be increased assuming the exhaust temperature remains the same?
A) 1065 C
B) 910 C
C) 1019 C
D) 973 C
E) 792 C
A) 1065 C
B) 910 C
C) 1019 C
D) 973 C
E) 792 C

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
28
In a nuclear power plant, heat is taken from the reactor core at 300ºC, work is done to drive an electric generator, and heat is rejected to the environment at 40ºC. What is the maximum possible thermal efficiency of this system?
A) 13%
B) 27%
C) 45%
D) 55%
E) 87%
A) 13%
B) 27%
C) 45%
D) 55%
E) 87%

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
29
An electric power plant generates 100 MW of power at an efficiency of 35%. At what rate must water be circulated pass the condenser if the change in water temperature is not to exceed 10ºC?
A) 2400 kg/s
B) 6800 kg/s
C) 3400 kg/s
D) 11300 kg/s
E) 4400 kg/s
A) 2400 kg/s
B) 6800 kg/s
C) 3400 kg/s
D) 11300 kg/s
E) 4400 kg/s

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
30
A steam engine with a boiler temperature of 227ºC and an exhaust temperature of 27ºC has a maximum efficiency of approximately
A) 67%
B) 88%
C) 14%
D) 12%
E) 40%
A) 67%
B) 88%
C) 14%
D) 12%
E) 40%

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
31
A steam engine operates between a high and low temperature of 550 C and 180 C. If the steam engine operates at 40% of its theoretical maximum efficiency and does work at a rate of 1000 W, calculate how much heat is discharged per hour.
A) 0.8 MJ/hr
B) 2.8 MJ/hr
C) 3.4 MJ/hr
D) 16 MJ/hr
E) 12 MJ/hr
A) 0.8 MJ/hr
B) 2.8 MJ/hr
C) 3.4 MJ/hr
D) 16 MJ/hr
E) 12 MJ/hr

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
32
Of the four steps in the Carnot cycle,
A) two are isothermal and two are isobaric.
B) two are adiabatic and two are isobaric.
C) two are isovolumic and two are isothermal.
D) two are adiabatic and two are isothermal.
E) two are adiabatic and two are isovolumic.
A) two are isothermal and two are isobaric.
B) two are adiabatic and two are isobaric.
C) two are isovolumic and two are isothermal.
D) two are adiabatic and two are isothermal.
E) two are adiabatic and two are isovolumic.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
33
A refrigerator has a coefficient of performance 4.0. How much heat is exhausted to the hot reservoir when 400 kJ of heat are removed from the cold reservoir?
A) 500 kJ
B) 400 kJ
C) 100 kJ
D) 80 kJ
E) Not enough information is given to answer this question.
A) 500 kJ
B) 400 kJ
C) 100 kJ
D) 80 kJ
E) Not enough information is given to answer this question.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
34
If a steam engine operates at half of its theoretical maximum efficiency (emax) and does work at a rate of W J/s, calculate how much heat is discharged per second.
A) W (1 -emax/2) / 2emax
B) 2W (1 - emax) / emax
C) W (1 - emax) / emax
D) W (2 - 2emax/2) / (emax/2)
E) 2W (1 -emax/2) / emax
A) W (1 -emax/2) / 2emax
B) 2W (1 - emax) / emax
C) W (1 - emax) / emax
D) W (2 - 2emax/2) / (emax/2)
E) 2W (1 -emax/2) / emax

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
35
What is the maximum possible efficiency of a steam engine operating between a high and low temperature of 550 C and 180 C?
A) 45%
B) 67%
C) 55%
D) 33%
E) 82%
A) 45%
B) 67%
C) 55%
D) 33%
E) 82%

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
36
The Carnot efficiency for a heat engine operating between the temperatures of 227º C and 27ºC is
A) 20%
B) 25%
C) 40%
D) 88%
E) 100%
A) 20%
B) 25%
C) 40%
D) 88%
E) 100%

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
37
You want to construct a perfect refrigerator from a perfect heat engine. What else do you need?
A) You need a second perfect heat engine.
B) You need a nonperfect heat engine.
C) You need a nonperfect refrigerator.
D) You need nothing else. A perfect refrigerator is just a perfect heat engine run backward.
E) A perfect refrigerator cannot be constructed even if you have a perfect heat engine.
A) You need a second perfect heat engine.
B) You need a nonperfect heat engine.
C) You need a nonperfect refrigerator.
D) You need nothing else. A perfect refrigerator is just a perfect heat engine run backward.
E) A perfect refrigerator cannot be constructed even if you have a perfect heat engine.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
38
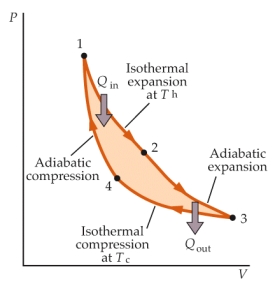 The schematic diagram above illustrates a Carnot engine. Which two paths of the cycle is work done by the gas?
The schematic diagram above illustrates a Carnot engine. Which two paths of the cycle is work done by the gas?A) 1 2, 2 3
B) 2 3, 3 4
C) 3 4, 4 1
D) 4 1, 1 2
E) only 1 2

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
39
A Carnot heat engine absorbs heat Q from a hot reservoir at 127ºC and exhausts heat to a cold reservoir at 27ºC. How much heat is exhausted to the cold reservoir?
A) Q
B) 27Q/127
C) 127Q/27
D) 3Q/4
E) 4Q/3
A) Q
B) 27Q/127
C) 127Q/27
D) 3Q/4
E) 4Q/3

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
40
A steam power plant with an efficiency of 65% of the maximum thermodynamic efficiency operates between 250º C and 40ºC. How much heat is rejected to the cold reservoir in doing 1.0 kJ of work?
A) 3.8 kJ
B) 0.83 kJ
C) 2.8 kJ
D) 1.8 kJ
E) 0.67 kJ
A) 3.8 kJ
B) 0.83 kJ
C) 2.8 kJ
D) 1.8 kJ
E) 0.67 kJ

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
41
A car of mass 2000 kg is traveling at 22.0 m/s on a day when the temperature is 20.0ºC. The driver steps on the brakes and stops the car. By how much does the entropy of the universe increase?
A) 1.6 kJ/K
B) 24 kJ/K
C) 4.8 105 J/K
D) 0.15 kJ/K
E) 3.3 kJ/K
A) 1.6 kJ/K
B) 24 kJ/K
C) 4.8 105 J/K
D) 0.15 kJ/K
E) 3.3 kJ/K

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
42
What is the entropy change when 4 kg of water at 0 C is frozen into 4 kg of ice at 0 C?
A) 4.9 kJ/K
B) an infinite change
C) -4.9 kJ/K
D) 1340 kJ/K
E) -33 kJ/K
A) 4.9 kJ/K
B) an infinite change
C) -4.9 kJ/K
D) 1340 kJ/K
E) -33 kJ/K

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
43
What is the maximum possible coefficient of performance of a heat pump that is capable of maintaining the interior of a house at +20ºC when the temperature outside is -40ºC?
A) 2.0
B) 5.2
C) 12
D) 4.9
E) 3.9
A) 2.0
B) 5.2
C) 12
D) 4.9
E) 3.9

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
44
Two moles of a gas at T = 350 K expand quasistatically and isothermally from an initial volume of 20 L to a final volume of 60 L. The change in entropy of the gas during this expansion is (R = 8.314 J/mol·K)
A) -17.4 J/K
B) 18.3 J/K
C) 20.4 J/K
D) -24.6 J/K
E) 27.8 J/K
A) -17.4 J/K
B) 18.3 J/K
C) 20.4 J/K
D) -24.6 J/K
E) 27.8 J/K

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
45
A container has 0.2 mole of O2 gas and the gas is heated from 0 C to 100 C. What is the change in entropy of the gas? (R = 8.314 J/mol·K)
A) 1.82 J/K
B) 1.30 J/K
C) 0.78 J/K
D) 26.8 J/K
E) zero
A) 1.82 J/K
B) 1.30 J/K
C) 0.78 J/K
D) 26.8 J/K
E) zero

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
46
When you make ice cubes, the entropy of the water
A) decreases.
B) remains unchanged.
C) increases.
D) is unchanged as the water cools but decreases as the water freezes.
E) decreases while the water is cooling but does not change as it turns to ice.
A) decreases.
B) remains unchanged.
C) increases.
D) is unchanged as the water cools but decreases as the water freezes.
E) decreases while the water is cooling but does not change as it turns to ice.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
47
The change in the entropy of the universe due to an operating Carnot engine
A) is zero.
B) must be positive.
C) must be negative.
D) could be positive or negative.
E) is meaningless to consider, because a Carnot engine has no connection to entropy.
A) is zero.
B) must be positive.
C) must be negative.
D) could be positive or negative.
E) is meaningless to consider, because a Carnot engine has no connection to entropy.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
48
A block of mass m = 0.2 kg slides across a rough horizontal surface with coefficient of kinetic friction k = 0.5. What is the change in entropy after the block has moved a distance of 1 m? The temperature of the block and surrounding is 22 C.
A) 4.5 -2 J/K
B) 3.3 -3 J/K
C) 6.7 10 -3 J/K
D) 9.0 10 -3 J/K
E) zero
A) 4.5 -2 J/K
B) 3.3 -3 J/K
C) 6.7 10 -3 J/K
D) 9.0 10 -3 J/K
E) zero

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
49
An ideal heat pump is used to pump head from the outside air at -5ºC to the hot-air supply for the heating fan in a house, which is at 35ºC. How much work is required to pump
1)5 kJ of heat into the house?
A) 0.165 kJ
B) 0.195 kJ
C) 0.205 kJ
D) 0.212 kJ
E) 0.224 kJ
1)5 kJ of heat into the house?
A) 0.165 kJ
B) 0.195 kJ
C) 0.205 kJ
D) 0.212 kJ
E) 0.224 kJ

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
50
Entropy is related to probability. An isolated system moves toward
A) a highly ordered state of low probability and high entropy.
B) a highly ordered state of high probability and high entropy.
C) a state of low order, high probability, and high entropy.
D) a state of low order, low probability, and high entropy.
E) a state of low order, high probability, and low entropy.
A) a highly ordered state of low probability and high entropy.
B) a highly ordered state of high probability and high entropy.
C) a state of low order, high probability, and high entropy.
D) a state of low order, low probability, and high entropy.
E) a state of low order, high probability, and low entropy.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
51
A heat pump needs to supply 20 kW of power to maintain the inside temperature of a house at 25 C while the outside temperature is 0 C. What is the power needed for the heat pump?
A) 3.36 kW
B) 2.42 kW
C) 2.78 kW
D) 4.12 kW
E) 1.68 kW
A) 3.36 kW
B) 2.42 kW
C) 2.78 kW
D) 4.12 kW
E) 1.68 kW

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
52
What is the maximum possible coefficient of performance of a heat pump that is capable of maintaining the interior of a house at 23ºC when the temperature outside is -30ºC?
A) 4.59
B) 3.27
C) 4.87
D) 2.63
E) 5.21
A) 4.59
B) 3.27
C) 4.87
D) 2.63
E) 5.21

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
53
A heat pump needs to supply 20 kW of power to maintain the inside temperature of a house at 25 C while the outside temperature is 0 C. What is the savings in the electric bill per month by using the heat pump instead of direct electrical heating? Assume that the heat pump/direct heating is on for 4 hours per day and 1 kW.hr costs $0.10.
A) $220
B) $20.2
C) $200
D) $40.4
E) $80.8
A) $220
B) $20.2
C) $200
D) $40.4
E) $80.8

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
54
Three moles of a gas at T = 250 K expand quasi-statically and adiabatically from an initial volume of 30 L to a final volume of 60 L. The change in entropy of the gas during this expansion is (R = 8.314 J/mol·K)
A) 17.3 J/K
B) 18.6 J/K
C) -17.4 J/K
D) 19.5 J/K
E) zero
A) 17.3 J/K
B) 18.6 J/K
C) -17.4 J/K
D) 19.5 J/K
E) zero

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
55
A thermodynamic system is taken in equilibrium steps from State I to State II. The quantities by which the process can be characterized are
1 internal energy,
2 entropy,
3 temperature,
4 work, and
5 heat.
Of these quantities, those that are independent of path are
A) 1, 2, and 3.
B) 2, 3, and 4.
C) 3, 4, and 5.
D) 1, 3, and 5.
E) 2, 3, and 5.
1 internal energy,
2 entropy,
3 temperature,
4 work, and
5 heat.
Of these quantities, those that are independent of path are
A) 1, 2, and 3.
B) 2, 3, and 4.
C) 3, 4, and 5.
D) 1, 3, and 5.
E) 2, 3, and 5.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
56
One mole of an ideal gas undergoes a reversible isothermal expansion from a volume of 1 L to a volume of 2 L. The change in entropy of the gas in terms of the universal gas constant R is
A) R/2
B) 2R
C) R ln(2)
D) R ln(½)
E) None of these is correct.
A) R/2
B) 2R
C) R ln(2)
D) R ln(½)
E) None of these is correct.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
57
A steam power plant with an efficiency of 65% of the maximum thermodynamic efficiency operates between 250º C and 40ºC. What is the change in the entropy of the universe when this plant does 1.0 kJ of work?
A) 16 J/K
B) 1.7 J/K
C) 55 J/K
D) 1.7 mJ/K
E) 0.85 J/K
A) 16 J/K
B) 1.7 J/K
C) 55 J/K
D) 1.7 mJ/K
E) 0.85 J/K

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following statements is true of an isolated system consisting of 15 gas molecules?
A) According to the second law, the entropy of the gas cannot decrease.
B) According to the second law, the entropy of the gas cannot increase.
C) According to the second law, the entropy of the gas is not likely to decrease.
D) According to the second law, the entropy of the gas is not likely to increase.
E) According to the second law, the entropy of the gas must stay the same.
A) According to the second law, the entropy of the gas cannot decrease.
B) According to the second law, the entropy of the gas cannot increase.
C) According to the second law, the entropy of the gas is not likely to decrease.
D) According to the second law, the entropy of the gas is not likely to increase.
E) According to the second law, the entropy of the gas must stay the same.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
59
A heat pump with COP = 5 uses electrical energy W to heat a house. How much heat is pumped into the house?
A) W
B) 5W
C) 6W
D) 4W
E) 1.2W
A) W
B) 5W
C) 6W
D) 4W
E) 1.2W

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
60
A quantity of heat is removed from a hot reservoir at absolute temperature T and then is added to a cold reservoir at absolute temperature T/2. The cold reservoir experiences an entropy increase S. What is the change in the entropy of the universe?
A) S
B) zero
C) -S
D) 2S
E) S/2
A) S
B) zero
C) -S
D) 2S
E) S/2

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
61
A box contains 10 red and 10 white marbles. The box is then given a good shake. What is the probability that all the 10 red marbles will be at the bottom of the box?
A) 1/1024
B) 1/2048
C) 1/512
D) 1/2
E) zero
A) 1/1024
B) 1/2048
C) 1/512
D) 1/2
E) zero

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck


