Exam 19: The Second Law of Thermodynamics
Exam 1: Systems of Measurement86 Questions
Exam 2: Motion in One Dimension103 Questions
Exam 3: Motion in Two and Three Dimensions67 Questions
Exam 4: Newtons Laws117 Questions
Exam 5: Applications of Newtons Laws75 Questions
Exam 6: Work and Energy71 Questions
Exam 7: Conservation of Energy73 Questions
Exam 8: Systems of Particles and Conservation of Linear Momentum107 Questions
Exam 9: Rotation119 Questions
Exam 10: Conservation of Angular Momentum67 Questions
Exam 11: Gravity90 Questions
Exam 12: Static Equilibrium and Elasticity65 Questions
Exam 13: Fluids91 Questions
Exam 14: Oscillations138 Questions
Exam 15: Wave Motion122 Questions
Exam 16: Superposition and Standing Waves125 Questions
Exam 17: Temperature and the Kinetic Theory of Gases85 Questions
Exam 18: Heat and the First Law of Thermodynamics114 Questions
Exam 19: The Second Law of Thermodynamics61 Questions
Exam 20: Thermal Properties and Processes54 Questions
Select questions type
Which of the following statements is true of an isolated system consisting of 15 gas molecules?
Free
(Multiple Choice)
4.8/5  (30)
(30)
Correct Answer:
C
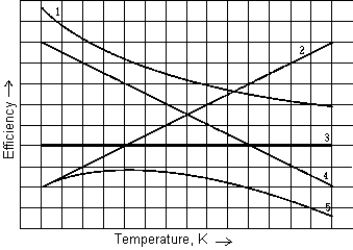 You can vary the efficiency of a Carnot engine by varying the temperature of the cold reservoir while maintaining the hot reservoir at constant temperature. The curve that best represents the efficiency of such an engine as a function of the temperature of the cold reservoir is
You can vary the efficiency of a Carnot engine by varying the temperature of the cold reservoir while maintaining the hot reservoir at constant temperature. The curve that best represents the efficiency of such an engine as a function of the temperature of the cold reservoir is
Free
(Multiple Choice)
4.9/5  (43)
(43)
Correct Answer:
D
A heat engine operating between the temperatures T1 and T2 takes in Q1 calories at temperature T1 and gives up Q2 calories at temperature T2. The efficiency of this heat engine is
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
B
Use the following diagram to answer the next problem. 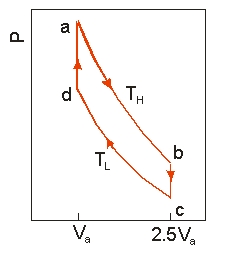 An ideal heat engine uses 0.01 mol of gas and operates between a hot reservoir at TH = 400 K and cold reservoir at TL = 300 K, in a cycle from a b c d a. From a b the gas undergoes an isothermal expansion, changing its volume from Va to 2.5Va. From b c, the pressure is reduced at a constant volume. From c d, the gas undergoes an isothermal compression, and from d a, the pressure is increased at a constant volume until the gas is back at the original condition at a.
-How much work is obtained from the engine in each cycle?
An ideal heat engine uses 0.01 mol of gas and operates between a hot reservoir at TH = 400 K and cold reservoir at TL = 300 K, in a cycle from a b c d a. From a b the gas undergoes an isothermal expansion, changing its volume from Va to 2.5Va. From b c, the pressure is reduced at a constant volume. From c d, the gas undergoes an isothermal compression, and from d a, the pressure is increased at a constant volume until the gas is back at the original condition at a.
-How much work is obtained from the engine in each cycle?
(Multiple Choice)
4.8/5  (31)
(31)
A refrigerator has a coefficient of performance 5.0. How much heat is exhausted to the hot reservoir when 200 kJ of heat are removed from the cold reservoir?
(Multiple Choice)
4.9/5  (29)
(29)
A refrigerator has a coefficient of performance 4.0. How much heat is exhausted to the hot reservoir when 400 kJ of heat are removed from the cold reservoir?
(Multiple Choice)
4.9/5  (33)
(33)
The efficiency of a Carnot engine operating between 300º C and 250ºC is about
(Multiple Choice)
4.8/5  (45)
(45)
A heat engine absorbs 64 kcal of heat from a hot reservoir and exhausts 42 kcal to a cold reservoir each cycle. Its efficiency is
(Multiple Choice)
4.9/5  (34)
(34)
A heat engine absorbs 70 kcal of heat from a hot reservoir and exhausts 50 kcal to a cold reservoir each cycle. Its efficiency is
(Multiple Choice)
4.8/5  (40)
(40)
A heat engine absorbs 150 J of heat from a hot reservoir and rejects 90 J to a cold reservoir. What is the efficiency of this engine?
(Multiple Choice)
4.9/5  (40)
(40)
The diagram below is a schematic of a heat engine. The three quantities, QH, QL, and W are represented, respectively, by 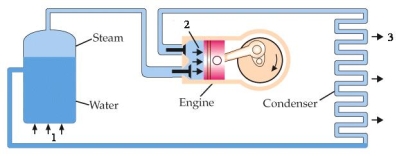
(Multiple Choice)
4.7/5  (36)
(36)
A refrigerator with a coefficient of performance 5.0 removes 25 kJ of heat from a cold reservoir. If this refrigerator is reversible and is run backward as a heat engine, what would be the efficiency of the heat engine?
(Multiple Choice)
4.9/5  (35)
(35)
Use the following diagram to answer the next problem.  An ideal heat engine uses 0.01 mol of gas and operates between a hot reservoir at TH = 400 K and cold reservoir at TL = 300 K, in a cycle from a b c d a. From a b the gas undergoes an isothermal expansion, changing its volume from Va to 2.5Va. From b c, the pressure is reduced at a constant volume. From c d, the gas undergoes an isothermal compression, and from d a, the pressure is increased at a constant volume until the gas is back at the original condition at a.
-If the engine operates at 50 cycles per second, the power output is
An ideal heat engine uses 0.01 mol of gas and operates between a hot reservoir at TH = 400 K and cold reservoir at TL = 300 K, in a cycle from a b c d a. From a b the gas undergoes an isothermal expansion, changing its volume from Va to 2.5Va. From b c, the pressure is reduced at a constant volume. From c d, the gas undergoes an isothermal compression, and from d a, the pressure is increased at a constant volume until the gas is back at the original condition at a.
-If the engine operates at 50 cycles per second, the power output is
(Multiple Choice)
4.9/5  (37)
(37)
We wish to increase the efficiency of an ideal heat engine from 25% to 35%. If the initial temperature of the hot reservoir is 650 C, to what temperature would this need to be increased assuming the exhaust temperature remains the same?
(Multiple Choice)
5.0/5  (36)
(36)
If you run a refrigerator in a closed room with the door to the refrigerator open, the temperature of the room
(Multiple Choice)
4.9/5  (28)
(28)
A steam power plant with an efficiency of 65% of the maximum thermodynamic efficiency operates between 250º C and 40ºC. How much heat is rejected to the cold reservoir in doing 1.0 kJ of work?
(Multiple Choice)
4.8/5  (35)
(35)
Two refrigerators, one with a COP of 4.0 and another with a COP of 5.0, both extract 400 kJ of heat from the cold reservoir (food). Calculate the difference in energy they exhaust to the hot reservoir and hence the room.
(Multiple Choice)
4.8/5  (35)
(35)
A heat pump needs to supply 20 kW of power to maintain the inside temperature of a house at 25 C while the outside temperature is 0 C. What is the power needed for the heat pump?
(Multiple Choice)
4.9/5  (30)
(30)
A steam power plant with an efficiency of 65% of the maximum thermodynamic efficiency operates between 250º C and 40ºC. What is the change in the entropy of the universe when this plant does 1.0 kJ of work?
(Multiple Choice)
4.9/5  (29)
(29)
Showing 1 - 20 of 61
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)