Deck 1: The Basics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/158
Play
Full screen (f)
Deck 1: The Basics
1
The greatest degree of ionic character is anticipated for the bond between
A)H and C.
B)H and Cl.
C)C and Cl.
D)H and Br.
E)Br and Cl.
A)H and C.
B)H and Cl.
C)C and Cl.
D)H and Br.
E)Br and Cl.
H and Cl.
2
Considering Lewis structures,which of these compounds possesses a single unpaired electron?
A)N2
B)N2O
C)NO
D)N2O4
E)O2
A)N2
B)N2O
C)NO
D)N2O4
E)O2
NO
3
Credit for the first synthesis of an organic compound from an inorganic precursor is usually given to ___.
A)Berzelius
B)Arrhenius
C)Kekule
D)Wöhler
E)Lewis
A)Berzelius
B)Arrhenius
C)Kekule
D)Wöhler
E)Lewis
Wöhler
4
Select the most electronegative element from the list below.
A)H
B)O
C)N
D)B
E)C
A)H
B)O
C)N
D)B
E)C

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is the Lewis structure for CH3CH2O2H?
A)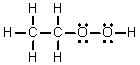
B)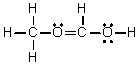
C)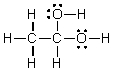
D)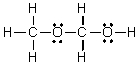
E)None of these choices.
A)

B)
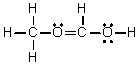
C)
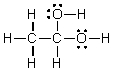
D)

E)None of these choices.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
6
What is the formal charge on carbon in the following structure? 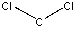
A)+2
B)+1
C)0
D)-1
E)-2

A)+2
B)+1
C)0
D)-1
E)-2

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
7
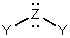 is a generalized structural representation which can be used for all of the following,except:
is a generalized structural representation which can be used for all of the following,except:A)H2O
B)H2Se
C)H2S
D)BeH2
E)There is no exception.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
8
Expansion of the valence shell to accommodate more than eight electrons is possible with ___.
A)fluorine
B)nitrogen
C)carbon
D)sulfur
E)beryllium
A)fluorine
B)nitrogen
C)carbon
D)sulfur
E)beryllium

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
9
Which is NOT a correct Lewis structure?
A)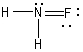
B)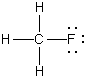
C)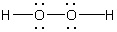
D)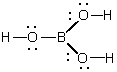
E)None of these choices.
A)
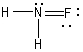
B)

C)
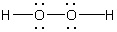
D)

E)None of these choices.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
10
Which of these is a correct electron-dot representation of the nitrite ion,NO2-? 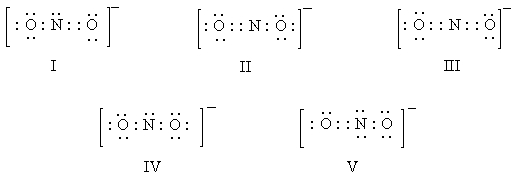
A)I
B)II
C)III
D)IV
E)V
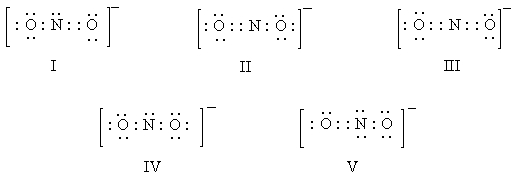
A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following best describes the origin of carbon atoms?
A)The Big Bang
B)amino acids found on meteorites
C)byproduct of chemical fusion in stars
D)gradual decay of radioactive isotopes of nitrogen
E)All of these choices.
A)The Big Bang
B)amino acids found on meteorites
C)byproduct of chemical fusion in stars
D)gradual decay of radioactive isotopes of nitrogen
E)All of these choices.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
12
In which structure(s)below does the oxygen have a formal charge of +1? 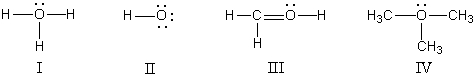 s
s
A)I only
B)II only
C)I and III
D)I and IV
E)I,III,and IV
 s
sA)I only
B)II only
C)I and III
D)I and IV
E)I,III,and IV

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
13
What is the formal charge on oxygen in the following structure? 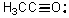
A)+2
B)+1
C)0
D)-1
E)-2
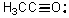
A)+2
B)+1
C)0
D)-1
E)-2

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
14
Which type of bonding is present in the compound CH3Li?
A)Ionic bonding
B)Covalent bonding
C)Hydrogen bonding
D)Ionic and covalent bonding
E)Ionic,covalent,and hydrogen bonding
A)Ionic bonding
B)Covalent bonding
C)Hydrogen bonding
D)Ionic and covalent bonding
E)Ionic,covalent,and hydrogen bonding

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following compounds contain a sulfur atom that bears a +1 formal charge?
A)H2S
B)SO2
C)SF6
D)MgSO4
E)H2SO4
A)H2S
B)SO2
C)SF6
D)MgSO4
E)H2SO4

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
16
Select the least electronegative element from the list below.
A)P
B)N
C)Mg
D)Si
E)K
A)P
B)N
C)Mg
D)Si
E)K

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
17
Which structure(s)contain(s)an oxygen that bears a formal charge of +1? 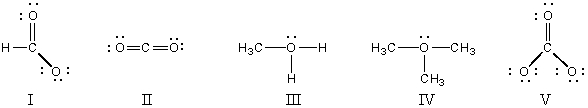
A)I and II
B)III and IV
C)V
D)II
E)I and V

A)I and II
B)III and IV
C)V
D)II
E)I and V

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
18
Which of these substances contain both covalent and ionic bonds?
A)NH4Cl
B)H2O2
C)CH4
D)HCN
E)H2S
A)NH4Cl
B)H2O2
C)CH4
D)HCN
E)H2S

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is the Lewis structure for CH3CO2H?
A)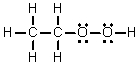
B)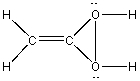
C)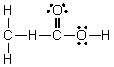
D)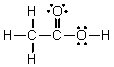
E)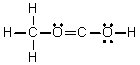
A)

B)

C)
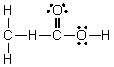
D)

E)
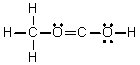

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
20
In which of the following does the central atom have 2 pairs of non-bonding electrons?
A)O3
B)CO2
C)CO32-
D)NH4+
E)H2S
A)O3
B)CO2
C)CO32-
D)NH4+
E)H2S

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
21
Which compound is not a constitutional isomer of the others? 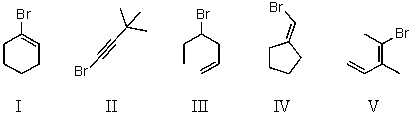
A)I and II
B)II
C)III
D)IV and V
E)All of these choices are isomers of each other.

A)I and II
B)II
C)III
D)IV and V
E)All of these choices are isomers of each other.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following represent pairs of constitutional isomers?
A)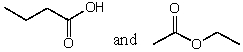
B)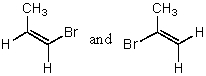
C)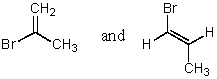
D)None of these pairs.
E)All of these pairs.
A)
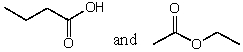
B)
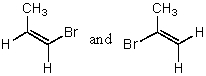
C)
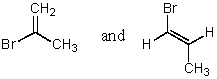
D)None of these pairs.
E)All of these pairs.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
23
In which of these cases does the central atom have a zero formal charge?
A)HFH
B)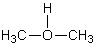
C)
D)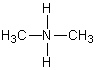
E)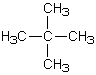
A)HFH
B)

C)
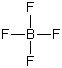
D)

E)


Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following represent a pair of constitutional isomers?
A)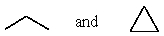
B)CH3CH=CH2 and CH2=CHCH3
C)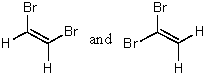
D)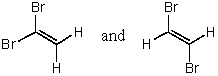
E)More than one of these choices.
A)
B)CH3CH=CH2 and CH2=CHCH3
C)
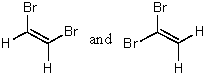
D)
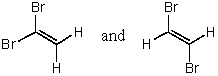
E)More than one of these choices.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
25
Which compound is not a constitutional isomer of the others? 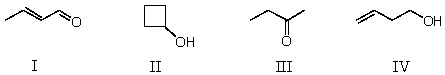
A)I
B)II
C)III
D)IV
E)All of these choices are isomers of each other.

A)I
B)II
C)III
D)IV
E)All of these choices are isomers of each other.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
26
Consider the following: CH3CH2CH=CHCH2CH3 CH3CH2CH2CH2CH=CH2
I II
CH3CH=CHCH2CH2CH3 CH2=CHCH2CH2CH2CH3
III IV
Which two structures represent the same compound?
A)I and II
B)II and III
C)I and III
D)II and IV
E)None of these choices.
I II
CH3CH=CHCH2CH2CH3 CH2=CHCH2CH2CH2CH3
III IV
Which two structures represent the same compound?
A)I and II
B)II and III
C)I and III
D)II and IV
E)None of these choices.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
27
What is the formal charge on oxygen in the following structure? 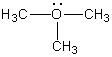
A)+2
B)+1
C)0
D)-1
E)-2

A)+2
B)+1
C)0
D)-1
E)-2

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following structures represent compounds that are constitutional isomers of each other? 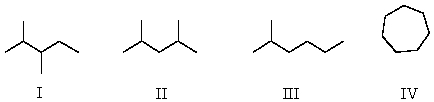
A)I and II
B)I and III
C)I,II,and III
D)I,II,III,and IV
E)II and III

A)I and II
B)I and III
C)I,II,and III
D)I,II,III,and IV
E)II and III

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
29
The formal charge on sulfur in sulfuric acid is: 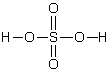
A)0
B)-1
C)+1
D)-2
E)+2
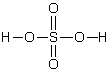
A)0
B)-1
C)+1
D)-2
E)+2

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following compounds is not a constitutional isomer of the others? 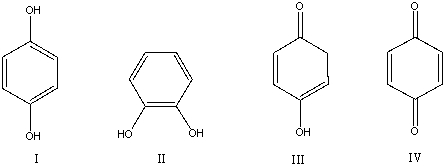
A)I
B)II
C)III
D)IV
E)All of these choices are constitutional isomers.

A)I
B)II
C)III
D)IV
E)All of these choices are constitutional isomers.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
31
In which structure(s)below does nitrogen have a formal charge of +1? 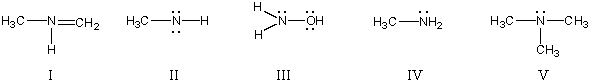
A)I
B)II and IV
C)III and V
D)I and V
E)V

A)I
B)II and IV
C)III and V
D)I and V
E)V

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
32
Listed below are electron dot formulas for several simple molecules and ions.All valence electrons are shown; however,electrical charges have been omitted deliberately. 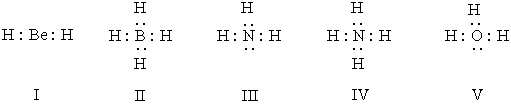 Which of the structures actually bear(s)a positive charge?
Which of the structures actually bear(s)a positive charge?
A)I
B)II
C)III
D)III and V
E)IV and V
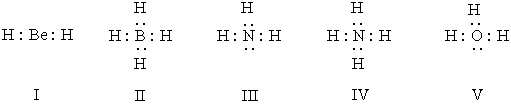 Which of the structures actually bear(s)a positive charge?
Which of the structures actually bear(s)a positive charge?A)I
B)II
C)III
D)III and V
E)IV and V

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is an ion with a negative one charge?
A)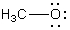
B)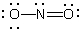
C)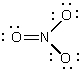
D)All of these choices.
E)None of these choices.
A)
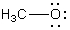
B)
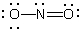
C)

D)All of these choices.
E)None of these choices.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following molecules or ions has a nitrogen with a formal charge of -1?
A)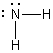
B)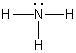
C)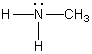
D)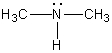
E)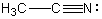
A)

B)

C)

D)

E)
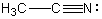

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
35
Which compound is not a constitutional isomer of the others? 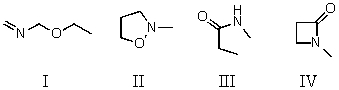
A)I
B)II
C)III
D)IV
E)All of these choices are isomers of each other.

A)I
B)II
C)III
D)IV
E)All of these choices are isomers of each other.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
36
CH3CH2OCH2CH3 and CH3CH2CH2CH2OH are examples of what are now termed:
A)Conformational isomers
B)Resonance structures
C)Functional isomers
D)Empirical isomers
E)Constitutional isomers
A)Conformational isomers
B)Resonance structures
C)Functional isomers
D)Empirical isomers
E)Constitutional isomers

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is a set of constitutional isomers? 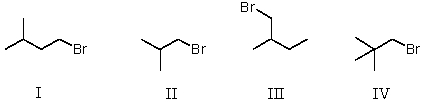
A)I and II
B)II and III
C)I,II,and III
D)II,III,and IV
E)I,III,and IV

A)I and II
B)II and III
C)I,II,and III
D)II,III,and IV
E)I,III,and IV

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
38
Which compound contains a nitrogen atom with a formal positive charge? 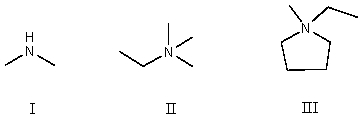
A)I
B)II
C)III
D)More than one of these choices.
E)None of these choices.

A)I
B)II
C)III
D)More than one of these choices.
E)None of these choices.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
39
Listed below are electron dot formulas for several simple molecules and ions.All valence electrons are shown; however,electrical charges have been omitted deliberately. 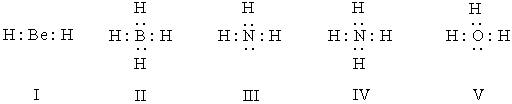 Which of the structures is negatively charged?
Which of the structures is negatively charged?
A)I
B)II
C)III
D)IV
E)V
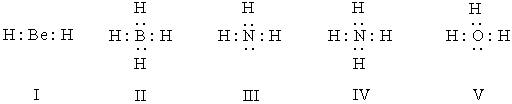 Which of the structures is negatively charged?
Which of the structures is negatively charged?A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following structure is an acceptable bond line formula for CH3CHClCHCH2? 
A)I
B)I and IV
C)II and III
D)I,II,and III
E)All of these choices.

A)I
B)I and IV
C)II and III
D)I,II,and III
E)All of these choices.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following species contributes more to the overall hybrid for the species in the box? 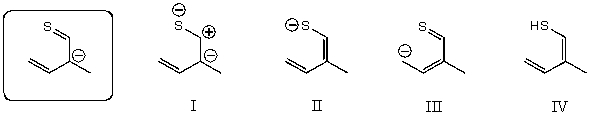
A)I
B)II
C)III
D)IV
E)The one in the box.

A)I
B)II
C)III
D)IV
E)The one in the box.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following species contributes more to the overall hybrid for the species in the box? 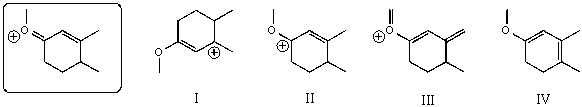
A)I
B)II
C)III
D)IV
E)The one in the box.

A)I
B)II
C)III
D)IV
E)The one in the box.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following species contributes more to the overall hybrid for the species in the box? 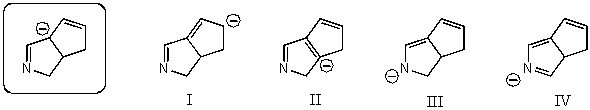
A)I
B)II
C)III
D)IV
E)The one in the box.

A)I
B)II
C)III
D)IV
E)The one in the box.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following could not be a resonance structure of CH3NO2?
A)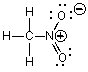
B)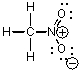
C)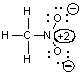
D)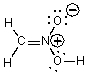
E)More than one of these choices.
A)

B)

C)

D)

E)More than one of these choices.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following species is a resonance form of the species in the box? 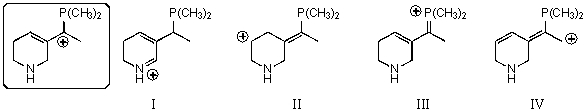
A)I
B)II
C)III
D)IV
E)None of these choices are correct resonance forms.

A)I
B)II
C)III
D)IV
E)None of these choices are correct resonance forms.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following species is/are a resonance form(s)of the species in the box? 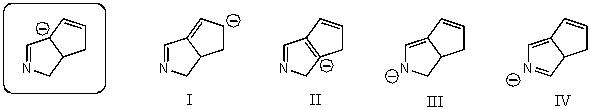
A)I and II
B)I and III
C)III and IV
D)III
E)More than two of these choices are correct resonance forms.

A)I and II
B)I and III
C)III and IV
D)III
E)More than two of these choices are correct resonance forms.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following structures is/are not a resonance form of the following species? 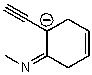
A)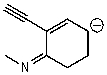
B)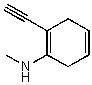
C)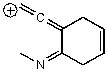
D)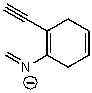
E)None of these choices are correct resonance forms.

A)

B)

C)

D)

E)None of these choices are correct resonance forms.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following species is/are not a resonance form(s)of the species in the box? 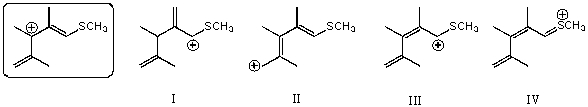
A)I
B)II
C)III
D)IV
E)More than two of these choices are incorrect resonance forms.

A)I
B)II
C)III
D)IV
E)More than two of these choices are incorrect resonance forms.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following species is/are not a resonance form(s)of the species in the box? 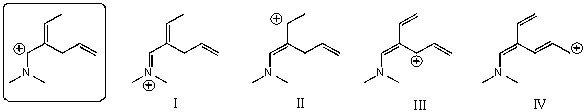
A)I and II
B)II and III
C)III and IV
D)I and IV
E)II and IV

A)I and II
B)II and III
C)III and IV
D)I and IV
E)II and IV

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following species is/are a resonance form(s)of the species in the box? 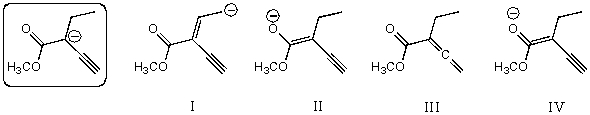
A)I and II
B)II and III
C)III
D)II
E)More than two of these choices are correct resonance forms.

A)I and II
B)II and III
C)III
D)II
E)More than two of these choices are correct resonance forms.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following species contributes more to the overall hybrid for the species in the box? 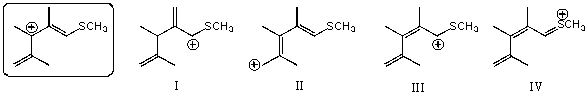
A)I
B)II
C)III
D)IV
E)The one in the box.

A)I
B)II
C)III
D)IV
E)The one in the box.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following is/are not a resonance form(s)of the species in the box? 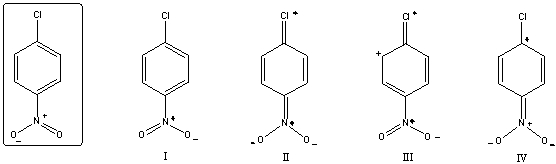
A)I
B)II
C)III
D)IV
E)More than two of these choices are incorrect resonance forms.

A)I
B)II
C)III
D)IV
E)More than two of these choices are incorrect resonance forms.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following species is/are not a resonance form(s)of the species in the box? 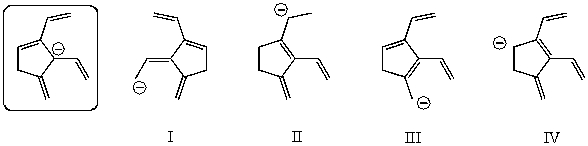
A)I
B)II
C)III
D)IV
E)More than two of these choices are incorrect resonance forms.

A)I
B)II
C)III
D)IV
E)More than two of these choices are incorrect resonance forms.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following species contributes more to the overall hybrid for the species in the box? 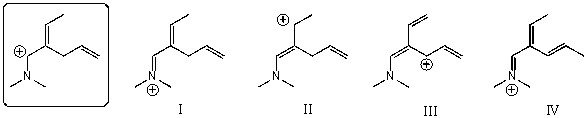
A)I
B)II
C)III
D)IV
E)The one in the box.

A)I
B)II
C)III
D)IV
E)The one in the box.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following species is a resonance form of the following species? 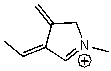
A)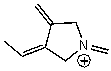
B)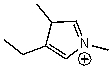
C)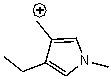
D)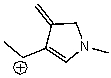
E)All of these choices are correct resonance forms.

A)

B)

C)

D)

E)All of these choices are correct resonance forms.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following species contributes more to the overall hybrid for the species in the box? 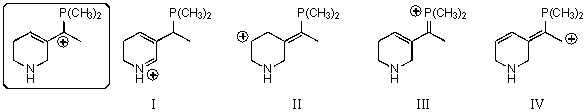
A)I
B)II
C)III
D)IV
E)The one in the box.

A)I
B)II
C)III
D)IV
E)The one in the box.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following pairs are NOT resonance structures?
A)
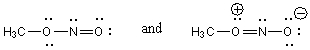
B)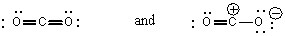
C)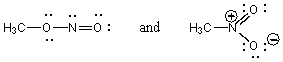
D)Each of these pairs represents resonance structures.
E)None of these pairs represents resonance structures.
A)
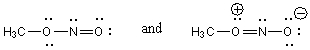
B)
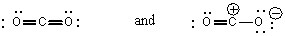
C)
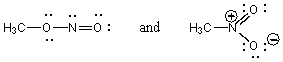
D)Each of these pairs represents resonance structures.
E)None of these pairs represents resonance structures.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following species are resonance forms of the species in the box? 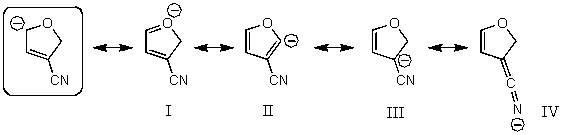
A)I and III
B)I and II
C)III and IV
D)II and IV
E)All of these choices are correct resonance forms.

A)I and III
B)I and II
C)III and IV
D)II and IV
E)All of these choices are correct resonance forms.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the structures below is not expected to contribute to the CO2 resonance hybrid?
A)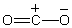
B)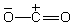
C)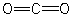
D)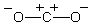
E)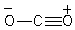
A)
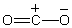
B)
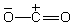
C)
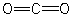
D)
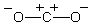
E)
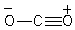

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following species is/are not a resonance form(s)of the anionic species in the box? 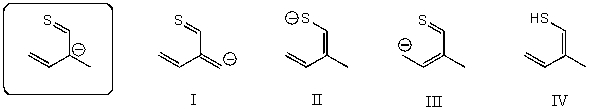
A)I
B)II and III
C)III and IV
D)I and IV
E)I and III

A)I
B)II and III
C)III and IV
D)I and IV
E)I and III

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following species contributes more to the overall hybrid for the species in the box? 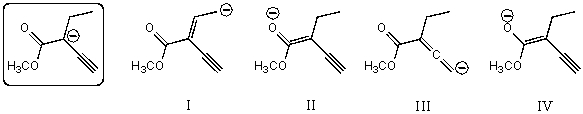
A)I
B)II
C)III
D)IV
E)The one in the box.

A)I
B)II
C)III
D)IV
E)The one in the box.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
62
Select the hybridized atomic orbital. 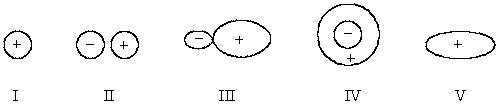
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
63
When the 1s orbitals of two hydrogen atoms combine to form a hydrogen molecule,how are the electrons distributed in the resulting molecular orbitals?
A)Two electrons in the bonding molecular orbital.
B)One electron in the bonding molecular orbital,one electron in the non-bonding molecular orbital.
C)One electron in the bonding molecular orbital,one electron in the antibonding molecular orbital.
D)Two electrons in the non-bonding molecular orbital.
E)Two electrons in the antibonding molecular orbital.
A)Two electrons in the bonding molecular orbital.
B)One electron in the bonding molecular orbital,one electron in the non-bonding molecular orbital.
C)One electron in the bonding molecular orbital,one electron in the antibonding molecular orbital.
D)Two electrons in the non-bonding molecular orbital.
E)Two electrons in the antibonding molecular orbital.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
64
Consider the following: CH3CH2CH2CH=CHCH2CH2CH3 CH3CH2CH2CH2CH2CH2CH=CH2
I II
CH3CH2CH=CHCH2CH2CH2CH3 CH2=CHCH2CH2CH2CH2CH2CH3
III IV
Which structures can exist as cis-trans isomers?
A)I and II
B)I and III
C)I and IV
D)II and III
E)I alone
I II
CH3CH2CH=CHCH2CH2CH2CH3 CH2=CHCH2CH2CH2CH2CH2CH3
III IV
Which structures can exist as cis-trans isomers?
A)I and II
B)I and III
C)I and IV
D)II and III
E)I alone

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
65
When two waves with equal amplitude and the opposite phase sign interact this results in generating
A)a wave that has half the amplitude of the original wave.
B)a wave that has double the amplitude of the original wave.
C)no wave as it has an amplitude of zero.
D)two new waves that have opposite phase signs of each other.
E)none of these choices.
A)a wave that has half the amplitude of the original wave.
B)a wave that has double the amplitude of the original wave.
C)no wave as it has an amplitude of zero.
D)two new waves that have opposite phase signs of each other.
E)none of these choices.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
66
How many sigma 1s-2sp3 bonds are there in ethane?
A)7
B)6
C)5
D)3
E)1
A)7
B)6
C)5
D)3
E)1

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
67
How many s-sp3 bonds are there in the following substance? 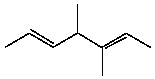
A)3
B)8
C)12
D)13
E)16

A)3
B)8
C)12
D)13
E)16

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
68
What point on the potential energy diagram below represents the most stable state for the hydrogen molecule? 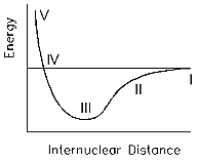
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
69
Cis-trans isomerism is possible only in the case of:
A)CH2=CBr2
B)CH2=CHBr
C)BrCH=CHBr
D)Br2C=CHBr
E)Br2C=CBr2
A)CH2=CBr2
B)CH2=CHBr
C)BrCH=CHBr
D)Br2C=CHBr
E)Br2C=CBr2

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
70
How many s-sp2 bonds are there in the following substance? 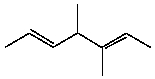
A)2
B)3
C)4
D)5
E)12

A)2
B)3
C)4
D)5
E)12

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
71
The C4-C5 carbon-carbon bond in the following molecule results from the overlap of which orbitals (in the order C4-C5)? 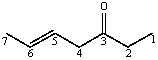
A)sp-sp2
B)sp-sp3
C)sp2-sp2
D)sp2-sp3
E)sp3-sp2

A)sp-sp2
B)sp-sp3
C)sp2-sp2
D)sp2-sp3
E)sp3-sp2

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
72
When the 1s orbitals of two hydrogen atoms combine to form a hydrogen molecule,how many molecular orbitals are formed?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
73
The relative probability of finding an electron a certain distance from the nucleus can be calculated using ___.
A)electron configuration
B)the wave function
C)statistical probability
D)atomic orbitals
E)all of these choices
A)electron configuration
B)the wave function
C)statistical probability
D)atomic orbitals
E)all of these choices

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
74
Which principle(s)or rule(s)must be used to determine the correct electronic configuration for carbon in its ground state?
A)Aufbau principle
B)Hund's Rule
C)Pauli exclusion principle
D)Aufbau principle and Hund's rule only
E)Aufbau principle,Hund's rule,and Pauli exclusion principle
A)Aufbau principle
B)Hund's Rule
C)Pauli exclusion principle
D)Aufbau principle and Hund's rule only
E)Aufbau principle,Hund's rule,and Pauli exclusion principle

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
75
In quantum mechanics a node (nodal surface or plane)is
A)a place where is negative.
B)a place where is positive.
C)a place where = 0.
D)a place where 2 is large.
E)a place where 2 is negative.
A)a place where is negative.
B)a place where is positive.
C)a place where = 0.
D)a place where 2 is large.
E)a place where 2 is negative.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
76
According to molecular orbital theory,in the case of a carbon-carbon double bond,the carbon-carbon bonding electrons of higher energy occupy this molecular orbital:
A)" bonding MO"
B)" bonding MO"
C)" * antibonding MO"
D)" * antibonding MO"
E)" * bonding MO"
A)" bonding MO"
B)" bonding MO"
C)" * antibonding MO"
D)" * antibonding MO"
E)" * bonding MO"

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
77
How many resonance structures can be written for the NO3- ion in which the nitrogen atom bears a formal charge of +1?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
78
When the 1s orbitals of two hydrogen atoms combine to form a hydrogen molecule,which molecular orbitals are formed?
A)One bonding molecular orbital only.
B)Two bonding molecular orbitals.
C)One bonding molecular orbital and one antibonding molecular orbital.
D)Two antibonding molecular orbitals.
E)Three bonding molecular orbitals.
A)One bonding molecular orbital only.
B)Two bonding molecular orbitals.
C)One bonding molecular orbital and one antibonding molecular orbital.
D)Two antibonding molecular orbitals.
E)Three bonding molecular orbitals.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following species exhibits resonance stabilization?
A)H2SO4
B)O3
C)CO2
D)CCl4
E)None of the species exhibit resonance.
A)H2SO4
B)O3
C)CO2
D)CCl4
E)None of the species exhibit resonance.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
80
According to molecular orbital theory,which molecule could not exist?
A)H2
B)He2
C)Li2
D)F2
E)N2
A)H2
B)He2
C)Li2
D)F2
E)N2

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck


