Exam 1: The Basics
Exam 1: The Basics158 Questions
Exam 2: Families of Carbon151 Questions
Exam 3: Acids and Bases149 Questions
Exam 4: Nomenclature and Conformations of Alkanes and Cycloalkanes163 Questions
Exam 5: Stereochemistry179 Questions
Exam 6: Nucleophilic Reactions91 Questions
Exam 7: Alkenes and Alkynes I220 Questions
Exam 8: Alkenes and Alkynes II163 Questions
Exam 9: Nuclear Magnetic Resonance and Mass Spectrometry158 Questions
Exam 10: Radical Reactions148 Questions
Exam 11: Alcohols and Ethers256 Questions
Exam 12: Alcohols From Carbonyl Compounds210 Questions
Exam 13: Conjugated Unsaturated Systems201 Questions
Exam 14: Aromatic Compounds186 Questions
Exam 15: Reactions of Aromatic Compounds207 Questions
Exam 16: Aldehydes and Ketones189 Questions
Exam 17: Carboxylic Acids and Their Derivatives213 Questions
Exam 18: Reactions at the Α Carbon of Carbonyl Compounds190 Questions
Exam 19: Condensation and Conjugate Addition Reactions of Carbonyl Compounds182 Questions
Exam 20: Amines207 Questions
Exam 21: Transition Metal Complexes86 Questions
Exam 22: Carbohydrates124 Questions
Exam 23: Lipids127 Questions
Exam 24: Amino Acids and Proteins135 Questions
Exam 25: Nucleic Acids and Protein Synthesis126 Questions
Select questions type
Which of these is a correct electron-dot representation of the nitrite ion,NO2-? 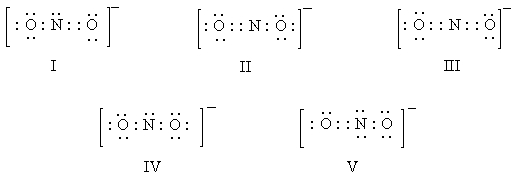
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
A
What is the hybridization of the C indicated with the arrow? 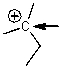
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
B
The greatest degree of ionic character is anticipated for the bond between
Free
(Multiple Choice)
4.9/5  (36)
(36)
Correct Answer:
B
What is the hybridization of the N atom in the following molecule? 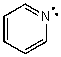
(Multiple Choice)
4.8/5  (30)
(30)
Listed below are electron dot formulas for several simple molecules and ions.All valence electrons are shown; however,electrical charges have been omitted deliberately. 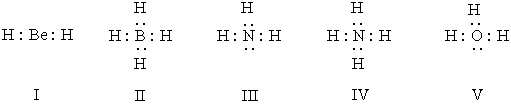 Which of the structures actually bear(s)a positive charge?
Which of the structures actually bear(s)a positive charge?
(Multiple Choice)
5.0/5  (45)
(45)
When the 1s orbitals of two hydrogen atoms combine to form a hydrogen molecule,how many molecular orbitals are formed?
(Multiple Choice)
4.8/5  (36)
(36)
Which of the structures below would be trigonal planar (a planar triangle)? (Electrical charges have been deliberately omitted.) 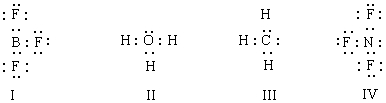
(Multiple Choice)
4.8/5  (35)
(35)
Which of these substances contain both covalent and ionic bonds?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following best describes the origin of carbon atoms?
(Multiple Choice)
4.8/5  (40)
(40)
Which compound is not a constitutional isomer of the others? 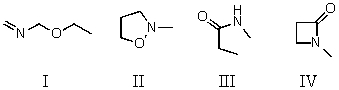
(Multiple Choice)
4.7/5  (38)
(38)
CH3CH2OCH2CH3 and CH3CH2CH2CH2OH are examples of what are now termed:
(Multiple Choice)
4.7/5  (38)
(38)
Identify the atomic orbital the lone pair electrons on the C atom are contained in: 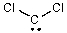
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following species is/are not a resonance form(s)of the species in the box? 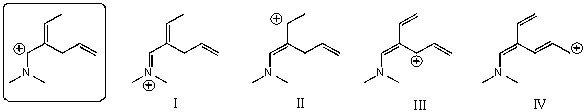
(Multiple Choice)
4.8/5  (41)
(41)
If a tetrahedral carbon atom were to lose its electrons from a single covalent bond its hybridization would change from sp3 hybridized to sp2 hybridized.
(True/False)
4.8/5  (34)
(34)
Which of the following represent a pair of constitutional isomers?
(Multiple Choice)
4.7/5  (35)
(35)
Consider the following: CH3CH2CH=CHCH2CH3 CH3CH2CH2CH2CH=CH2
I II
CH3CH=CHCH2CH2CH3 CH2=CHCH2CH2CH2CH3
III IV
Which two structures represent the same compound?
(Multiple Choice)
4.9/5  (36)
(36)
When the 1s orbitals of two hydrogen atoms combine to form a hydrogen molecule,which molecular orbitals are formed?
(Multiple Choice)
4.7/5  (30)
(30)
Showing 1 - 20 of 158
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)