Deck 3: Acids and Bases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/149
Play
Full screen (f)
Deck 3: Acids and Bases
1
Which of these is not a Lewis acid?
A)AlBr3
B)BCl3
C)Ph3P
D)D+
E)All these choices are Lewis acids
A)AlBr3
B)BCl3
C)Ph3P
D)D+
E)All these choices are Lewis acids
Ph3P
2
Which of the following is a Lewis acid?
A)H3O+
B)BF3
C)NF3
D)OH-
E)More than one of these choices.
A)H3O+
B)BF3
C)NF3
D)OH-
E)More than one of these choices.
More than one of these choices.
3
Which of these is not a Lewis acid?
A)AlCl3
B)H3O+
C)FeCl3
D)SO3
E)C4H10
A)AlCl3
B)H3O+
C)FeCl3
D)SO3
E)C4H10
C4H10
4
Which of the following is not a Lewis base?
A)NH3
B)H-
C)BF3
D)H2O
E)H3C-
A)NH3
B)H-
C)BF3
D)H2O
E)H3C-

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
5
Consider the equilibrium  Which are the Bronsted-Lowry bases?
Which are the Bronsted-Lowry bases?
A)PO43- and HPO42-
B)PO43- and OH-
C)PO43- and H2O
D)H2O and OH-
E)H2O and HPO42-
 Which are the Bronsted-Lowry bases?
Which are the Bronsted-Lowry bases?A)PO43- and HPO42-
B)PO43- and OH-
C)PO43- and H2O
D)H2O and OH-
E)H2O and HPO42-

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
6
According to the Lewis definition,a base is a(n)___.
A)proton donor
B)electron pair donor
C)hydroxide ion donor
D)hydrogen ion donor
E)electron pair acceptor
A)proton donor
B)electron pair donor
C)hydroxide ion donor
D)hydrogen ion donor
E)electron pair acceptor

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
7
This species is a carbon-based Lewis acid:
A)CH4
B)HCCl3
C)CH3+
D):CH3-
E)·CH3
A)CH4
B)HCCl3
C)CH3+
D):CH3-
E)·CH3

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
8
What is/are the product(s)of the following acid-base mechanism? 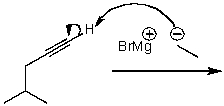
A)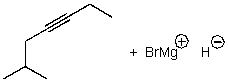
B)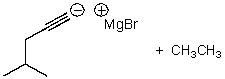
C)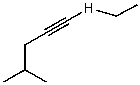
D)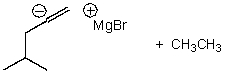
E)None of these choices.

A)

B)
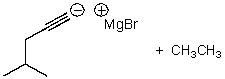
C)

D)
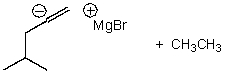
E)None of these choices.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is not a conjugate acid - conjugate base pair (in that order)?
A)H3PO4,H2PO4-
B)HBF4,BF4-
C)CH3CH2OH,CH3CH2O--
D)H3O+,H2O
E)HPO42-,H2PO4-
A)H3PO4,H2PO4-
B)HBF4,BF4-
C)CH3CH2OH,CH3CH2O--
D)H3O+,H2O
E)HPO42-,H2PO4-

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
10
What is the conjugate base of ethanol?
A)CH3CH2O-
B)CH3CH2-
C)CH3CH2OH2+
D)CH3CH3
E)CH3OCH3
A)CH3CH2O-
B)CH3CH2-
C)CH3CH2OH2+
D)CH3CH3
E)CH3OCH3

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
11
What is/are the product(s)of the following acid-base mechanism? 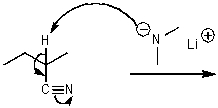
A)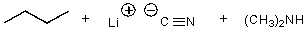
B)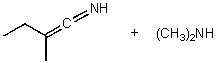
C)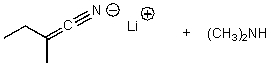
D)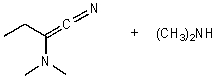
E)None of these choices.

A)
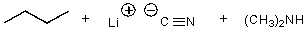
B)
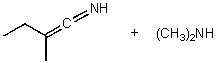
C)
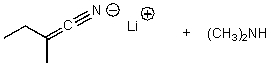
D)
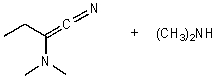
E)None of these choices.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
12
What is/are the product(s)of the following acid-base mechanism? 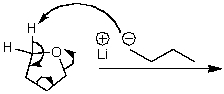
A)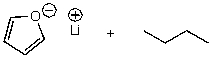
B)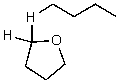
C)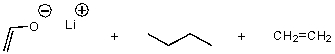
D)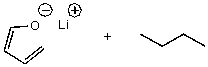
E)None of these choices.

A)
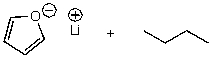
B)

C)

D)
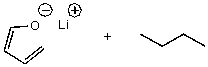
E)None of these choices.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
13
A conjugate base of sulfuric acid is:
A)H3SO4+
B)SO3
C)HSO4-
D)H2SO3
E)HSO3-
A)H3SO4+
B)SO3
C)HSO4-
D)H2SO3
E)HSO3-

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
14
Which pair of species are both bases in the following reaction? 
A)H2O and CN-
B)H3O+ and H2O
C)HCN and H3O+
D)HCN and CN-
E)H3O+ and CN-

A)H2O and CN-
B)H3O+ and H2O
C)HCN and H3O+
D)HCN and CN-
E)H3O+ and CN-

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
15
What is/are the product(s)of the following acid-base mechanism? 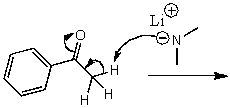
A)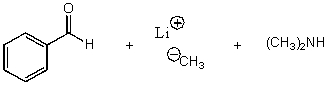
B)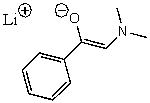
C)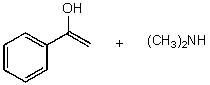
D)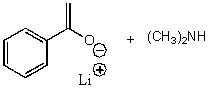
E)None of these choices.

A)
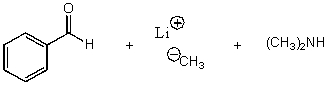
B)

C)

D)

E)None of these choices.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is not a Bronsted-Lowry acid?
A)H2O
B)(CH3)3N
C)NH4+
D)CH3CO2H
E)HC CH
A)H2O
B)(CH3)3N
C)NH4+
D)CH3CO2H
E)HC CH

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
17
Which of these is not a true statement?
A)All Lewis bases are also Bronsted-Lowry bases.
B)All Lewis acids contain hydrogen.
C)All Bronsted-Lowry acids contain hydrogen.
D)All Lewis acids are electron deficient.
E)According to the Bronsted-Lowry theory,water is both an acid and a base.
A)All Lewis bases are also Bronsted-Lowry bases.
B)All Lewis acids contain hydrogen.
C)All Bronsted-Lowry acids contain hydrogen.
D)All Lewis acids are electron deficient.
E)According to the Bronsted-Lowry theory,water is both an acid and a base.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
18
What is/are the product(s)of the following acid-base mechanism? 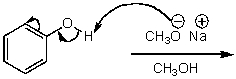
A)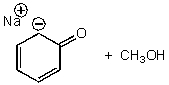
B)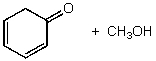
C)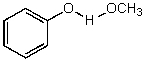
D)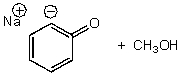
E)None of these choices.

A)

B)
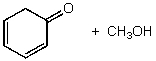
C)

D)

E)None of these choices.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
19
What is/are the product(s)of the following acid-base mechanism? 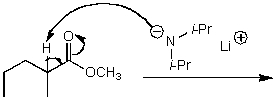
A)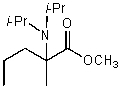
B)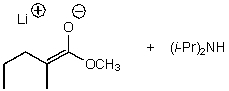
C)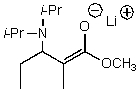
D)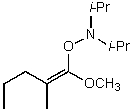
E)None of these choices.

A)

B)

C)

D)

E)None of these choices.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
20
What is/are the product(s)of the following acid-base mechanism? 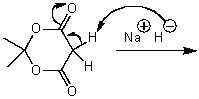
A)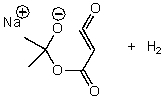
B)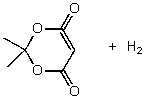
C)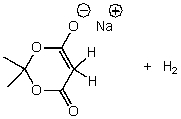
D)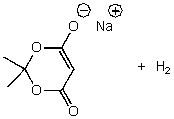
E)None of these choices.

A)

B)

C)

D)

E)None of these choices.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
21
Which of these phosphorus-based acids is diprotic?
A)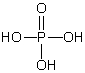
B)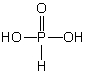
C)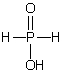
D)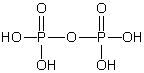
E)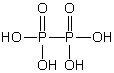
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
22
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 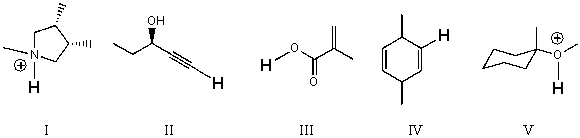
A)I > II > III > IV > V
B)III > V > II > I > IV
C)V > II > IV > III > I
D)III > I > V > II > IV
E)V > III > I > II > IV

A)I > II > III > IV > V
B)III > V > II > I > IV
C)V > II > IV > III > I
D)III > I > V > II > IV
E)V > III > I > II > IV

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
23
What is the pH of a 1.0 M aqueous solution of CH3COOH given that its pKa value is 4.75?
A)0
B)0.0021
C)0.0042
D)2.4
E)2.7
A)0
B)0.0021
C)0.0042
D)2.4
E)2.7

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
24
In the following reaction which chemical species is acting like a nucleophile? 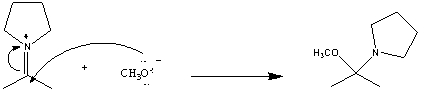
A)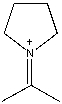
B)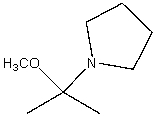
C)CH3O-
D)two of these choices
E)there is no nucleophile in this reaction

A)

B)

C)CH3O-
D)two of these choices
E)there is no nucleophile in this reaction

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
25
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 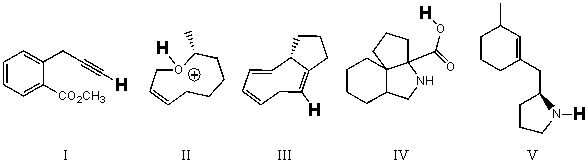
A)I > IV > II > V > III
B)II > IV > I > V > III
C)II > IV > V > I > III
D)IV > I > II > V > III
E)IV > II > I > V > III

A)I > IV > II > V > III
B)II > IV > I > V > III
C)II > IV > V > I > III
D)IV > I > II > V > III
E)IV > II > I > V > III

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following organic compounds is the strongest acid?
A)C6H12 pKa = 52
B)CH3CH3 pKa = 50
C)CH3CH2OH pKa = 18
D)CH3CO2H pKa = 5
E)CF3CO2H pKa = 0
A)C6H12 pKa = 52
B)CH3CH3 pKa = 50
C)CH3CH2OH pKa = 18
D)CH3CO2H pKa = 5
E)CF3CO2H pKa = 0

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
27
In the following reaction,which chemical species is acting like an electrophile? 
A)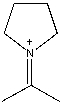
B)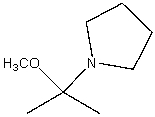
C)CH3O-
D)two of these choices
E)there is no electrophile in this reaction

A)

B)

C)CH3O-
D)two of these choices
E)there is no electrophile in this reaction

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following organic compounds has the strongest conjugate base?
A)C6H12 pKa = 52
B)CH3CH3 pKa = 50
C)CH3CH2OH pKa = 18
D)CH3CO2H pKa = 5
E)CF3CO2H pKa = 0
A)C6H12 pKa = 52
B)CH3CH3 pKa = 50
C)CH3CH2OH pKa = 18
D)CH3CO2H pKa = 5
E)CF3CO2H pKa = 0

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following correctly lists the compounds in order of decreasing acidity?
A)H2O > HC CH > NH3 > CH3CH3
B)HC H > H2O > NH3 > CH3CH3
C)CH3CH3 > HC CH > NH3 > H2O
D)CH3CH3 > HC CH > H2O > NH3
E)H2O > NH3 > HC CH > CH3CH3
A)H2O > HC CH > NH3 > CH3CH3
B)HC H > H2O > NH3 > CH3CH3
C)CH3CH3 > HC CH > NH3 > H2O
D)CH3CH3 > HC CH > H2O > NH3
E)H2O > NH3 > HC CH > CH3CH3

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
30
Which is the strongest acid?
A)CH3CH2OH
B)CH3CO2H
C)HC CH
D)CH2=CH2
E)CH3CH3
A)CH3CH2OH
B)CH3CO2H
C)HC CH
D)CH2=CH2
E)CH3CH3

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
31
The reaction between which combination of substances below cannot be classified as a Bronsted-Lowry acid-base reaction?
A)CH3Li + C2H5OH
B)H2SO4 + CH3CO2Na
C)BF3 + NH3
D)H3O+ + CH3NH-
E)two of these choices
A)CH3Li + C2H5OH
B)H2SO4 + CH3CO2Na
C)BF3 + NH3
D)H3O+ + CH3NH-
E)two of these choices

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
32
For the equilibrium  the two substances which are both acids are:
the two substances which are both acids are:
A)H2O and H3O+
B)CH3NH3+ and H2O
C)CH3NH3+ and CH3NH2
D)CH3NH3+ and H3O+
E)CH3NH2 and H2O
 the two substances which are both acids are:
the two substances which are both acids are:A)H2O and H3O+
B)CH3NH3+ and H2O
C)CH3NH3+ and CH3NH2
D)CH3NH3+ and H3O+
E)CH3NH2 and H2O

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
33
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 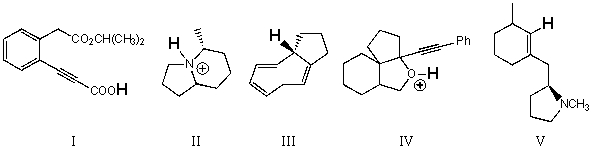
A)I > IV > II > V > III
B)II > I > IV > V > III
C)V > III > II > I > IV
D)IV > I > II > V > III
E)IV > II > I > V > III

A)I > IV > II > V > III
B)II > I > IV > V > III
C)V > III > II > I > IV
D)IV > I > II > V > III
E)IV > II > I > V > III

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
34
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 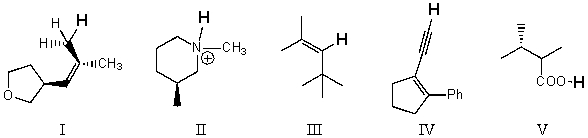
A)I > II > III > IV > V
B)V > IV > II > III > I
C)V > II > IV > III > I
D)II > V > IV > III > I
E)V > IV > III > II > I

A)I > II > III > IV > V
B)V > IV > II > III > I
C)V > II > IV > III > I
D)II > V > IV > III > I
E)V > IV > III > II > I

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
35
Rank the following hydrogen atoms from highest to lowest in acidity in the structure shown below. 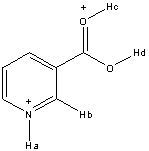
A)Ha > Hb > Hc > Hd
B)Ha > Hc > Hd > Hb
C)Hc > Hd > Ha > Hb
D)Hd > Ha > Hc > Hb
E)Hc > Ha > Hd > Hb

A)Ha > Hb > Hc > Hd
B)Ha > Hc > Hd > Hb
C)Hc > Hd > Ha > Hb
D)Hd > Ha > Hc > Hb
E)Hc > Ha > Hd > Hb

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
36
The acidity constant,Ka,differs from the equilibrium constant,Keq,for the dissociation of the same acid in water at the same temperature and concentration in what way?
A)Ka can be determined experimentally with less accuracy than Keq.
B)The two terms are identical numerically.
C)Ka is used for strong acids only; Keq for weak acids.
D)Ka is the reciprocal of Keq.
E)Keq = Ka/[H2O].
A)Ka can be determined experimentally with less accuracy than Keq.
B)The two terms are identical numerically.
C)Ka is used for strong acids only; Keq for weak acids.
D)Ka is the reciprocal of Keq.
E)Keq = Ka/[H2O].

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
37
Hydrogen atom(s)from which position(s)is (are)most likely to be abstracted when the following substance is treated with NaH? 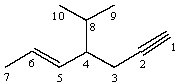
A)1
B)1,5,6
C)1,2
D)3,4,7,8,9,10
E)Hydrogens from all of the positions are equally likely to be abstracted by NaH.

A)1
B)1,5,6
C)1,2
D)3,4,7,8,9,10
E)Hydrogens from all of the positions are equally likely to be abstracted by NaH.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
38
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 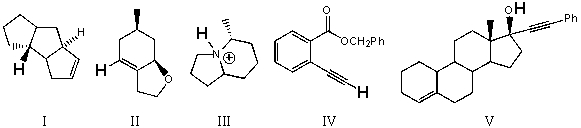
A)III > V > IV > II > I
B)V > IV > III > II > I
C)V > III > IV > II > I
D)III > V > II > IV > I
E)IV > II > I > V > III

A)III > V > IV > II > I
B)V > IV > III > II > I
C)V > III > IV > II > I
D)III > V > II > IV > I
E)IV > II > I > V > III

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
39
Which of these is not a diprotic acid?
A)H2S
B)H2SO4
C)(COOH)2
D)H2PO4-
E)All of these choices are diprotic.
A)H2S
B)H2SO4
C)(COOH)2
D)H2PO4-
E)All of these choices are diprotic.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following substances has a hydrogen atom with pKa ≈ 25?
A)CH3CH2CH2CH2CO2H
B)CH3CHCH2C≡CCH3
C)CH3CH2CH2C≡CH
D)CH3CH2CH2CH2CH=CH2
E)CH3CH2CH2CH2NH2
A)CH3CH2CH2CH2CO2H
B)CH3CHCH2C≡CCH3
C)CH3CH2CH2C≡CH
D)CH3CH2CH2CH2CH=CH2
E)CH3CH2CH2CH2NH2

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
41
The basic species are arranged in decreasing order of basicity in the sequence:
A)F- > OCH3- > NH2- > CH3CH2-
B)OCH3- > CH3CH2- > NH2- > F-
C)CH3CH2- > NH2- > OCH3- > F-
D)NH2- > CH3CH2- > F- > OCH3-
E)NH2- > OCH3- > CH3CH2- > F-
A)F- > OCH3- > NH2- > CH3CH2-
B)OCH3- > CH3CH2- > NH2- > F-
C)CH3CH2- > NH2- > OCH3- > F-
D)NH2- > CH3CH2- > F- > OCH3-
E)NH2- > OCH3- > CH3CH2- > F-

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
42
Which is an incorrect statement?
A)RSH compounds are stronger acids than ROH compounds.
B)PH3 is a weaker base than NH3.
C)NH2- is a stronger base than OH-.
D)OH- is a stronger base than OR-.
E)H- is a stronger base than OR-.
A)RSH compounds are stronger acids than ROH compounds.
B)PH3 is a weaker base than NH3.
C)NH2- is a stronger base than OH-.
D)OH- is a stronger base than OR-.
E)H- is a stronger base than OR-.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
43
Which set contains non-equivalent members?
A)enthalpy and heat content
B)endothermic reaction and + H
C)exothermic reaction and - H
D)kinetic energy and energy of motion
E)high energy and high stability
A)enthalpy and heat content
B)endothermic reaction and + H
C)exothermic reaction and - H
D)kinetic energy and energy of motion
E)high energy and high stability

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
44
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 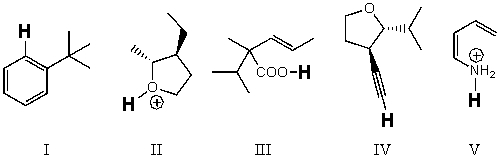
A)III > II > IV > V > I
B)II > III > V > IV > I
C)II > V > III > IV > I
D)V > III > II > IV > I
E)I > IV > II > III > V

A)III > II > IV > V > I
B)II > III > V > IV > I
C)II > V > III > IV > I
D)V > III > II > IV > I
E)I > IV > II > III > V

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
45
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 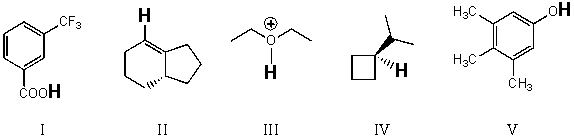
A)I > II > III > IV > V
B)III > V > II > I > IV
C)V > II > IV > III > I
D)III > I > V > II > IV
E)V > III > I > II > IV

A)I > II > III > IV > V
B)III > V > II > I > IV
C)V > II > IV > III > I
D)III > I > V > II > IV
E)V > III > I > II > IV

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
46
Which one of the following is a true statement?
A)The stronger the acid,the larger is its pKa.
B)The conjugate base of a strong acid is a strong base.
C)Acid-base reactions always favor the formation of the stronger acid and the stronger base.
D)Strong acids can have negative pKa values.
E)Hydrogen need not be present in the molecular formula of a Bronsted-Lowry acid.
A)The stronger the acid,the larger is its pKa.
B)The conjugate base of a strong acid is a strong base.
C)Acid-base reactions always favor the formation of the stronger acid and the stronger base.
D)Strong acids can have negative pKa values.
E)Hydrogen need not be present in the molecular formula of a Bronsted-Lowry acid.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
47
The correct sequence of the ions shown,in order of increasing basicity,is:
A)CH3CH2:- < CH2=CH:- < HC C:-
B)CH3CH2:- < HC C:- < CH2=CH:-
C)HC C:- < CH3CH2:- < CH2=CH:-
D)CH2=CH:- < HC C:- < CH3CH2:-
E)HC C:- < CH2=CH:- < CH3CH2:-
A)CH3CH2:- < CH2=CH:- < HC C:-
B)CH3CH2:- < HC C:- < CH2=CH:-
C)HC C:- < CH3CH2:- < CH2=CH:-
D)CH2=CH:- < HC C:- < CH3CH2:-
E)HC C:- < CH2=CH:- < CH3CH2:-

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
48
When proton transfer reactions reach equilibrium,there have been formed
A)the weaker acid and the weaker base.
B)the weaker acid and the stronger base.
C)the stronger acid and the weaker base.
D)the stronger acid and the stronger base.
E)All proton transfers go to completion; they are not equilibrium processes.
A)the weaker acid and the weaker base.
B)the weaker acid and the stronger base.
C)the stronger acid and the weaker base.
D)the stronger acid and the stronger base.
E)All proton transfers go to completion; they are not equilibrium processes.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the acids below would have the strongest conjugate base?
A)CH3CH2OH pKa = 18
B)CH3CO2H pKa = 4.75
C)ClCH2CO2H pKa = 2.81
D)Cl2CHCO2H pKa = 1.29
E)Cl3CCO2H pKa = 0.66
A)CH3CH2OH pKa = 18
B)CH3CO2H pKa = 4.75
C)ClCH2CO2H pKa = 2.81
D)Cl2CHCO2H pKa = 1.29
E)Cl3CCO2H pKa = 0.66

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
50
Based on the position of the central atom in the periodic chart,we predict that the strongest acid of the following is:
A)H2O
B)H2S
C)H2Se
D)H2Te
E)All acids are equal in strength.
A)H2O
B)H2S
C)H2Se
D)H2Te
E)All acids are equal in strength.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
51
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 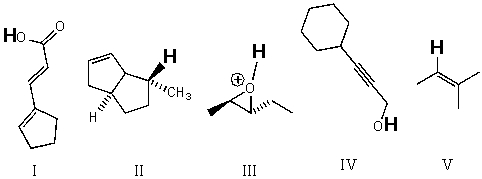
A)III > IV > I > V > II
B)II > III > V > IV > I
C)II > V > III > IV > I
D)V > III > II > IV > I
E)III > I > IV > V > II

A)III > IV > I > V > II
B)II > III > V > IV > I
C)II > V > III > IV > I
D)V > III > II > IV > I
E)III > I > IV > V > II

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
52
Why cannot one determine the relative acid strengths of HClO4 and HNO3 using aqueous solutions of these acids?
A)The acids are insufficiently soluble for the measurements.
B)A more basic solvent than H2O must be used.
C)H2O is too basic a solvent for the distinction to be made.
D)These oxidizing acids cause redox reactions to occur.
E)Actually,the acid strengths can be determined using aqueous solutions.
A)The acids are insufficiently soluble for the measurements.
B)A more basic solvent than H2O must be used.
C)H2O is too basic a solvent for the distinction to be made.
D)These oxidizing acids cause redox reactions to occur.
E)Actually,the acid strengths can be determined using aqueous solutions.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
53
Select the strongest base.
A)OH-
B)RC C-
C)NH2-
D)CH2=CH-
E)CH3CH2-
A)OH-
B)RC C-
C)NH2-
D)CH2=CH-
E)CH3CH2-

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
54
A group of acids arranged in order of decreasing acidity is: HNO3 > CH3COOH > C6H5OH > H2O > HC CH
What is the arrangement of the conjugate bases of these compounds in decreasing order of basicity?
A)NO3- > CH3COO- > C6H5O- > OH- > HC C-
B)CH3COO- > C6H5O- > NO3- > OH- > HC C-
C)C6H5O- > NO3- > HC C- > OH- > CH3COO-
D)HC C- > OH- > C6H5O- > CH3COO- > NO3-
E)No prediction of relative base strength is possible.
What is the arrangement of the conjugate bases of these compounds in decreasing order of basicity?
A)NO3- > CH3COO- > C6H5O- > OH- > HC C-
B)CH3COO- > C6H5O- > NO3- > OH- > HC C-
C)C6H5O- > NO3- > HC C- > OH- > CH3COO-
D)HC C- > OH- > C6H5O- > CH3COO- > NO3-
E)No prediction of relative base strength is possible.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
55
The compounds ethane,ethene,and ethyne exhibit this order of increasing acidity:
A)Ethyne < ethene < ethane
B)Ethene < ethyne < ethane
C)Ethane < ethyne < ethene
D)Ethane < ethene < ethyne
E)Ethene < ethane < ethyne
A)Ethyne < ethene < ethane
B)Ethene < ethyne < ethane
C)Ethane < ethyne < ethene
D)Ethane < ethene < ethyne
E)Ethene < ethane < ethyne

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
56
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 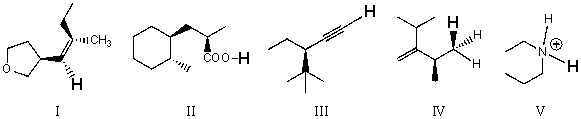
A)I > II > III > IV > V
B)II > V > III > I > IV
C)II > III > V > I > IV
D)III > I > V > II > IV
E)V > II > III > I > IV

A)I > II > III > IV > V
B)II > V > III > I > IV
C)II > III > V > I > IV
D)III > I > V > II > IV
E)V > II > III > I > IV

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
57
The compound aniline,C6H5NH2,has weakly basic properties in aqueous solution.In this other solvent,aniline would behave as a strong base.
A)CH3OH
B)CH3CH2OH
C)CF3CO2H
D)Liquid NH3
E)CH3(CH2)4CH3
A)CH3OH
B)CH3CH2OH
C)CF3CO2H
D)Liquid NH3
E)CH3(CH2)4CH3

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
58
What prediction can be made of the relative strengths of the conjugate bases of: H2S,HCl,SiH4,PH3?
A)PH2- > SiH3- > HS- > Cl-
B)SiH3- > PH2- > HS- > Cl-
C)Cl- > HS- > PH2- > SiH3-
D)HS- > Cl- > SiH3- > PH2-
E)Cl- > PH2- > SiH3- > HS-
A)PH2- > SiH3- > HS- > Cl-
B)SiH3- > PH2- > HS- > Cl-
C)Cl- > HS- > PH2- > SiH3-
D)HS- > Cl- > SiH3- > PH2-
E)Cl- > PH2- > SiH3- > HS-

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
59
For the simple hydrides,MHn,pKa values decrease in the order:
A)CH4 > NH3 > H2O > H2S > HBr
B)HBr > H2S > H2O > NH3 > CH4
C)HBr > H2O > NH3 > H2S > CH4
D)NH3 > H2S > CH4 > H2O > HBr
E)H2S > H2O > HBr > NH3 > CH4
A)CH4 > NH3 > H2O > H2S > HBr
B)HBr > H2S > H2O > NH3 > CH4
C)HBr > H2O > NH3 > H2S > CH4
D)NH3 > H2S > CH4 > H2O > HBr
E)H2S > H2O > HBr > NH3 > CH4

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
60
An acid,HA,has the following thermodynamic values for its dissociation in water at 27 ºC: H = -8.0 kJ mol-1; S = -70 J K-1mol-1.The G for the process is:
A)+29 kJ mol-1
B)+13 kJ mol-1
C)-6.1 kJ mol-1
D)-13 kJ mol-1
E)-29 kJ mol-1
A)+29 kJ mol-1
B)+13 kJ mol-1
C)-6.1 kJ mol-1
D)-13 kJ mol-1
E)-29 kJ mol-1

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
61
For the following acid-base reaction,which statement is true taking S into consideration? 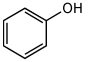
A)The reaction is an exothermic reaction and S is positive.
B)The reaction is an exothermic reaction and S is approximately zero.
C)The reaction is an endothermic reaction and S is positive.
D)The reaction is an endothermic reaction and S is approximately zero.
E)None of these choices.


A)The reaction is an exothermic reaction and S is positive.
B)The reaction is an exothermic reaction and S is approximately zero.
C)The reaction is an endothermic reaction and S is positive.
D)The reaction is an endothermic reaction and S is approximately zero.
E)None of these choices.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
62
Which is the weakest acid?
A)CH3CH2CH2CH2CHFCO2H
B)CH3CHCH2CH2CH2CH2OH
C)CH3CH2CH2CH2CH2SO3H
D)CH3CH2CH2CH2CH=CH2
E)CH3CH2CH2CH2NH2
A)CH3CH2CH2CH2CHFCO2H
B)CH3CHCH2CH2CH2CH2OH
C)CH3CH2CH2CH2CH2SO3H
D)CH3CH2CH2CH2CH=CH2
E)CH3CH2CH2CH2NH2

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
63
For the following acid/base reaction which statement is true taking S into consideration? 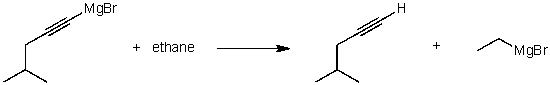
A)The reaction is an exothermic reaction and S is approximately zero.
B)The reaction is an endothermic reaction and S is negative.
C)The reaction is an exothermic reaction and S is negative.
D)The reaction is an exothermic reaction and S is positive.
E)None of these choices.

A)The reaction is an exothermic reaction and S is approximately zero.
B)The reaction is an endothermic reaction and S is negative.
C)The reaction is an exothermic reaction and S is negative.
D)The reaction is an exothermic reaction and S is positive.
E)None of these choices.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
64
For the following acid / base reaction which statement is true taking H into consideration? 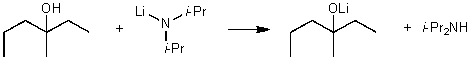
A)The reaction is an exothermic reaction and H is approximately zero.
B)The reaction is an endothermic reaction and H is negative.
C)The reaction is an exothermic reaction and H is negative.
D)The reaction is an exothermic reaction and H is positive.
E)None of these choices.

A)The reaction is an exothermic reaction and H is approximately zero.
B)The reaction is an endothermic reaction and H is negative.
C)The reaction is an exothermic reaction and H is negative.
D)The reaction is an exothermic reaction and H is positive.
E)None of these choices.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
65
For the following acid/base reaction which statement is true taking S into consideration? 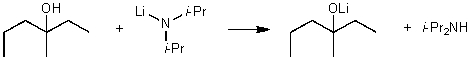
A)The reaction is an endothermic reaction and S is approximately zero.
B)The reaction is an endothermic reaction and S is negative.
C)The reaction is an exothermic reaction and S is negative.
D)The reaction is an exothermic reaction and S is approximately zero.
E)None of these choices.

A)The reaction is an endothermic reaction and S is approximately zero.
B)The reaction is an endothermic reaction and S is negative.
C)The reaction is an exothermic reaction and S is negative.
D)The reaction is an exothermic reaction and S is approximately zero.
E)None of these choices.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
66
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 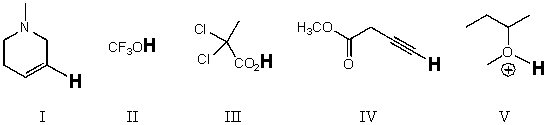
A)V > III > II > IV > I
B)II > V > III > I > IV
C)II > III > V > IV > I
D)III > I > V > II > IV
E)V > II > III > I > IV

A)V > III > II > IV > I
B)II > V > III > I > IV
C)II > III > V > IV > I
D)III > I > V > II > IV
E)V > II > III > I > IV

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
67
For the following acid-base reaction,which statement is true taking S into consideration? 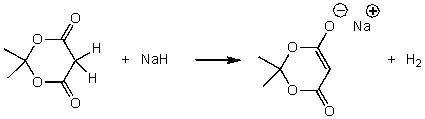
A)The reaction is an exothermic reaction and S is positive.
B)The reaction is an exothermic reaction and S is negative.
C)The reaction is an endothermic reaction and S is positive.
D)The reaction is an endothermic reaction and S is approximately zero.
E)None of these choices.

A)The reaction is an exothermic reaction and S is positive.
B)The reaction is an exothermic reaction and S is negative.
C)The reaction is an endothermic reaction and S is positive.
D)The reaction is an endothermic reaction and S is approximately zero.
E)None of these choices.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
68
For the following acid / base reaction which statement is true taking S into consideration? 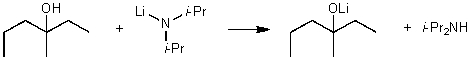
A)The reaction is an endothermic reaction and S is approximately zero.
B)The reaction is an endothermic reaction and S is negative.
C)The reaction is an exothermic reaction and S is negative.
D)The reaction is an exothermic reaction and S is approximately zero.
E)None of these choices.

A)The reaction is an endothermic reaction and S is approximately zero.
B)The reaction is an endothermic reaction and S is negative.
C)The reaction is an exothermic reaction and S is negative.
D)The reaction is an exothermic reaction and S is approximately zero.
E)None of these choices.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
69
For the following acid / base reaction which statement is true taking H into consideration? 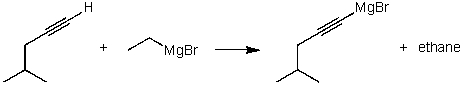
A)The reaction is an exothermic reaction and H is approximately zero.
B)The reaction is an endothermic reaction and H is negative.
C)The reaction is an exothermic reaction and H is negative.
D)The reaction is an exothermic reaction and H is positive.
E)None of these choices.

A)The reaction is an exothermic reaction and H is approximately zero.
B)The reaction is an endothermic reaction and H is negative.
C)The reaction is an exothermic reaction and H is negative.
D)The reaction is an exothermic reaction and H is positive.
E)None of these choices.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
70
Which is the strongest acid?
A)CH2ClCH2CH2CH2CH2CO2H
B)CH3CHBrCH2CH2CH2CO2H
C)CH3CH2CH2CBr2CH2CO2H
D)CH3CH2CH2CHFCH2CO2H
E)CH3CH2CH2CF2CH2CO2H
A)CH2ClCH2CH2CH2CH2CO2H
B)CH3CHBrCH2CH2CH2CO2H
C)CH3CH2CH2CBr2CH2CO2H
D)CH3CH2CH2CHFCH2CO2H
E)CH3CH2CH2CF2CH2CO2H

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
71
For the following acid/base reaction which statement is true taking S into consideration? 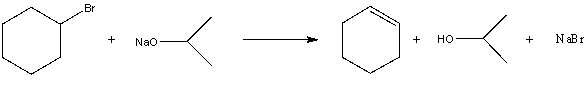
A)" G is negative and S is approximately zero"
B)" G is negative and S is negative"
C)" G is positive and S is positive"
D)" G is negative and S is positive"
E)"None of these choices."

A)" G is negative and S is approximately zero"
B)" G is negative and S is negative"
C)" G is positive and S is positive"
D)" G is negative and S is positive"
E)"None of these choices."

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
72
For the following acid/base reaction which statement is true taking S into consideration? 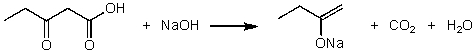
A)The reaction is an exothermic reaction and S is approximately zero.
B)The reaction is an endothermic reaction and S is negative.
C)The reaction is an exothermic reaction and S is negative.
D)The reaction is an exothermic reaction and S is positive.
E)None of these choices.

A)The reaction is an exothermic reaction and S is approximately zero.
B)The reaction is an endothermic reaction and S is negative.
C)The reaction is an exothermic reaction and S is negative.
D)The reaction is an exothermic reaction and S is positive.
E)None of these choices.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
73
For the following acid-base reaction,which statement is true taking H into consideration? 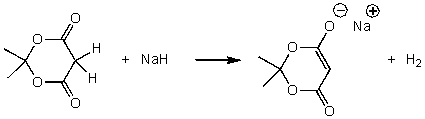
A)The reaction is an exothermic reaction and H is approximately zero.
B)The reaction is an endothermic reaction and H is negative.
C)The reaction is an endothermic reaction and H is positive.
D)The reaction is an exothermic reaction and H is negative.
E)None of these choices.

A)The reaction is an exothermic reaction and H is approximately zero.
B)The reaction is an endothermic reaction and H is negative.
C)The reaction is an endothermic reaction and H is positive.
D)The reaction is an exothermic reaction and H is negative.
E)None of these choices.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
74
For the following acid-base reaction,which statement is true taking S into consideration? 
A)" G is negative and S is approximately zero"
B)" G is positive and S is approximately zero"
C)" G is positive and S is negative"
D)" G is negative and S is positive"
E)"None of these choices."

A)" G is negative and S is approximately zero"
B)" G is positive and S is approximately zero"
C)" G is positive and S is negative"
D)" G is negative and S is positive"
E)"None of these choices."

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
75
For the following acid-base reaction,which statement is true taking H into consideration? 
A)The reaction is an exothermic reaction and H is approximately zero.
B)The reaction is an endothermic reaction and H is negative.
C)The reaction is an exothermic reaction and H is negative.
D)The reaction is an endothermic reaction and H is positive.
E)None of these choices.

A)The reaction is an exothermic reaction and H is approximately zero.
B)The reaction is an endothermic reaction and H is negative.
C)The reaction is an exothermic reaction and H is negative.
D)The reaction is an endothermic reaction and H is positive.
E)None of these choices.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
76
For the following acid/base reaction which statement is true taking S into consideration? 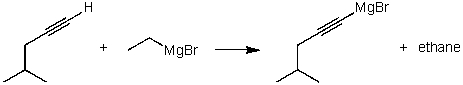
A)The reaction is an exothermic reaction and S is approximately zero.
B)The reaction is an endothermic reaction and S is negative.
C)The reaction is an exothermic reaction and S is negative.
D)The reaction is an exothermic reaction and S is positive.
E)None of these choices.

A)The reaction is an exothermic reaction and S is approximately zero.
B)The reaction is an endothermic reaction and S is negative.
C)The reaction is an exothermic reaction and S is negative.
D)The reaction is an exothermic reaction and S is positive.
E)None of these choices.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
77
Which is the strongest acid?
A)CH3CH2CH2CH2CHFCO2H
B)CH3CHBrCH2CH2CH2CO2H
C)CH3CH2CH2CHClCH2CO2H
D)CH3CH2CH2CHFCH2CO2H
E)CH3CH2CH2CHICH2CO2H
A)CH3CH2CH2CH2CHFCO2H
B)CH3CHBrCH2CH2CH2CO2H
C)CH3CH2CH2CHClCH2CO2H
D)CH3CH2CH2CHFCH2CO2H
E)CH3CH2CH2CHICH2CO2H

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following is an untrue statement?
A)The % dissociation of a weak acid increases with increasing dilution of the acid solution.
B)The stronger an acid,the weaker its conjugate base.
C)The larger the value of Ka for an acid,the smaller the value of its pKa.
D)Comparison of the acidity of strong acids in solution requires the use of a solvent less basic than water.
E)The stronger the acid,the more positive the value of Gº for the dissociation.
A)The % dissociation of a weak acid increases with increasing dilution of the acid solution.
B)The stronger an acid,the weaker its conjugate base.
C)The larger the value of Ka for an acid,the smaller the value of its pKa.
D)Comparison of the acidity of strong acids in solution requires the use of a solvent less basic than water.
E)The stronger the acid,the more positive the value of Gº for the dissociation.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
79
For the following acid/base reaction which statement is true taking S into consideration? 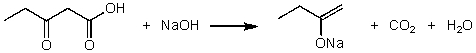
A)" G is negative and S is approximately zero"
B)" G is negative and S is negative"
C)" G is positive and S is positive"
D)" G is negative and S is positive"
E)"None of these choices."

A)" G is negative and S is approximately zero"
B)" G is negative and S is negative"
C)" G is positive and S is positive"
D)" G is negative and S is positive"
E)"None of these choices."

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
80
For the following acid / base reaction which statement is true taking H into consideration? 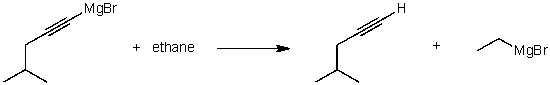
A)The reaction is an exothermic reaction and H is approximately zero.
B)The reaction is an endothermic reaction and H is negative.
C)The reaction is an exothermic reaction and H is negative.
D)The reaction is an exothermic reaction and H is positive.
E)None of these choices.

A)The reaction is an exothermic reaction and H is approximately zero.
B)The reaction is an endothermic reaction and H is negative.
C)The reaction is an exothermic reaction and H is negative.
D)The reaction is an exothermic reaction and H is positive.
E)None of these choices.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck


