Exam 3: Acids and Bases
Exam 1: The Basics158 Questions
Exam 2: Families of Carbon151 Questions
Exam 3: Acids and Bases149 Questions
Exam 4: Nomenclature and Conformations of Alkanes and Cycloalkanes163 Questions
Exam 5: Stereochemistry179 Questions
Exam 6: Nucleophilic Reactions91 Questions
Exam 7: Alkenes and Alkynes I220 Questions
Exam 8: Alkenes and Alkynes II163 Questions
Exam 9: Nuclear Magnetic Resonance and Mass Spectrometry158 Questions
Exam 10: Radical Reactions148 Questions
Exam 11: Alcohols and Ethers256 Questions
Exam 12: Alcohols From Carbonyl Compounds210 Questions
Exam 13: Conjugated Unsaturated Systems201 Questions
Exam 14: Aromatic Compounds186 Questions
Exam 15: Reactions of Aromatic Compounds207 Questions
Exam 16: Aldehydes and Ketones189 Questions
Exam 17: Carboxylic Acids and Their Derivatives213 Questions
Exam 18: Reactions at the Α Carbon of Carbonyl Compounds190 Questions
Exam 19: Condensation and Conjugate Addition Reactions of Carbonyl Compounds182 Questions
Exam 20: Amines207 Questions
Exam 21: Transition Metal Complexes86 Questions
Exam 22: Carbohydrates124 Questions
Exam 23: Lipids127 Questions
Exam 24: Amino Acids and Proteins135 Questions
Exam 25: Nucleic Acids and Protein Synthesis126 Questions
Select questions type
Which one of the following is a true statement?
Free
(Multiple Choice)
4.9/5  (39)
(39)
Correct Answer:
D
Which of the acids below would have the strongest conjugate base?
Free
(Multiple Choice)
4.7/5  (28)
(28)
Correct Answer:
A
According to Bronsted-Lowry theory,an acid is a substance that can ___.
Free
(Short Answer)
4.9/5  (44)
(44)
Correct Answer:
donate a proton
The strongest acid that can exist in an aqueous solution is the hydronium ion H3O+.
(True/False)
4.9/5  (33)
(33)
Write an equation that shows the reaction between acetic acid (CH3COOH)and triethylamine (CH3CH2)3N.Draw all non-bonding lone electron pairs and show the electron flow with curved arrows.
(Essay)
4.8/5  (28)
(28)
Which reaction of these potential acids and bases does not occur to any appreciable degree due to an unfavorable equilibrium?
(Multiple Choice)
5.0/5  (37)
(37)
In the following reaction,which chemical species is acting like an electrophile? 
(Multiple Choice)
5.0/5  (37)
(37)
What prediction can be made of the relative strengths of the conjugate bases of: H2S,HCl,SiH4,PH3?
(Multiple Choice)
4.9/5  (37)
(37)
For the following acid / base reaction which statement is true taking S into consideration? 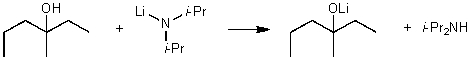
(Multiple Choice)
4.8/5  (35)
(35)
What is/are the product(s)of the following acid-base mechanism? 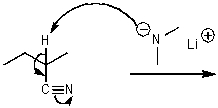
(Multiple Choice)
4.8/5  (39)
(39)
For the following acid/base reaction which statement is true taking S into consideration? 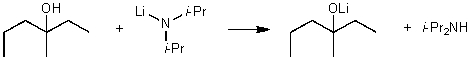
(Multiple Choice)
4.9/5  (47)
(47)
For the following acid / base reaction which statement is true taking H into consideration? 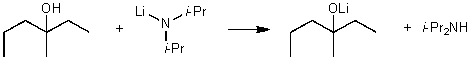
(Multiple Choice)
4.9/5  (49)
(49)
What compounds are produced when NaSH is added to a mixture of water and methanol?
(Multiple Choice)
4.8/5  (38)
(38)
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 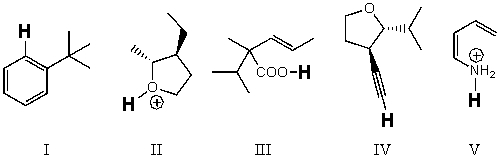
(Multiple Choice)
4.9/5  (36)
(36)
For the following acid/base reaction which statement is true taking S into consideration? 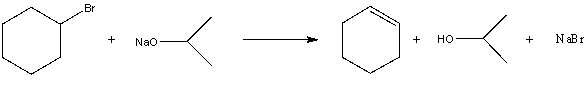
(Multiple Choice)
4.7/5  (39)
(39)
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 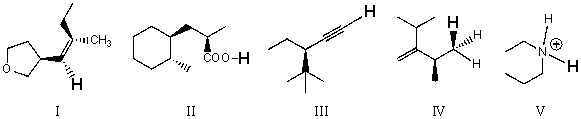
(Multiple Choice)
4.9/5  (35)
(35)
As a consequence of the "leveling effect," the strongest acid which can exist in appreciable concentration in aqueous solution is:
(Multiple Choice)
4.8/5  (26)
(26)
Which of the reaction conditions would not afford the following transformation? 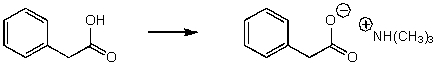
(Multiple Choice)
4.8/5  (34)
(34)
Showing 1 - 20 of 149
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)