Deck 13: Enzymeskinetics and Specificity
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/50
Play
Full screen (f)
Deck 13: Enzymeskinetics and Specificity
1
All are distinctive features of enzymes EXCEPT:
A) regulation.
B) catalytic activity.
C) ability to change ΔG.
D) specificity.
E) none is true.
A) regulation.
B) catalytic activity.
C) ability to change ΔG.
D) specificity.
E) none is true.
C
2
All are true for catalysts EXCEPT:
A) They work by lowering the energy of activation.
B) The average energy of the reaction is unchanged.
C) They combine transiently with the reactants promoting a reactive transition state condition.
D) They are regenerated after each reaction cycle.
E) All are true.
A) They work by lowering the energy of activation.
B) The average energy of the reaction is unchanged.
C) They combine transiently with the reactants promoting a reactive transition state condition.
D) They are regenerated after each reaction cycle.
E) All are true.
E
3
The free energy of activation, ΔG  , is defined as:
, is defined as:
A) The average free energy of the product formed.
B) The rate of a chemical reaction in relationship to the concentration of reactant molecules.
C) The energy required to raise the average energy of one mole of reactant to the transition state energy.
D) The amount of energy released by a spontaneous reaction.
E) The lowest point on a free energy diagram.
 , is defined as:
, is defined as:A) The average free energy of the product formed.
B) The rate of a chemical reaction in relationship to the concentration of reactant molecules.
C) The energy required to raise the average energy of one mole of reactant to the transition state energy.
D) The amount of energy released by a spontaneous reaction.
E) The lowest point on a free energy diagram.
C
4
Which of the following is true regarding the Briggs and Haldane steady state assumption?
A) It is defined by the equation

B) It states the rate of enzyme-substrate complex formation differs from the rate of enzyme-substrate disappearance.
C) The concentration of the enzyme-substrate complex reaches a constant value even in a dynamic system.
D) The enzyme-substrate complex will always dissociate to form E + P.
E) The total amount of enzyme is variable, depending on the amount of substrate available.
A) It is defined by the equation

B) It states the rate of enzyme-substrate complex formation differs from the rate of enzyme-substrate disappearance.
C) The concentration of the enzyme-substrate complex reaches a constant value even in a dynamic system.
D) The enzyme-substrate complex will always dissociate to form E + P.
E) The total amount of enzyme is variable, depending on the amount of substrate available.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
5
The specific site on the enzyme where ____ binds and catalysis occurs is called the ____ site.
A) coenzyme; substrate
B) substrate; active
C) coenzyme; regulatory
D) regulatory; active
E) none of the above
A) coenzyme; substrate
B) substrate; active
C) coenzyme; regulatory
D) regulatory; active
E) none of the above

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
6
All of the following are true statements about the transition state of a reaction EXCEPT:
A) The transition state is not an appropriate indication of the rate of a reaction.
B) The transition state is located at the height of a free energy diagram.
C) The energy required to raise the average energy of one mole of reactant to the transition state is the free energy of activation.
D) Reaching the transition state indicates that there is a high probability that the reaction will occur.
E) The transition state energy level is the sum of the energy levels of the reactants and products.
A) The transition state is not an appropriate indication of the rate of a reaction.
B) The transition state is located at the height of a free energy diagram.
C) The energy required to raise the average energy of one mole of reactant to the transition state is the free energy of activation.
D) Reaching the transition state indicates that there is a high probability that the reaction will occur.
E) The transition state energy level is the sum of the energy levels of the reactants and products.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
7
What reaction would NOT proceed via bimolecular elementary steps?
A) C + D → T + U
B) A reaction with a rate constant in the units of s−1.
C) 2A → D + E
D) A reaction with a molecularity of 2.
E) A reaction with a rate constant in the units of M−1s−1.
A) C + D → T + U
B) A reaction with a rate constant in the units of s−1.
C) 2A → D + E
D) A reaction with a molecularity of 2.
E) A reaction with a rate constant in the units of M−1s−1.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
8
All of the following are properties of a coenzyme EXCEPT:
A) They are usually actively involved in the catalytic reaction of the enzyme.
B) They tend to be stable to heat.
C) They can serve as intermediate carriers of functional groups.
D) They are protein components.
E) They may contain vitamins as part of their structure.
A) They are usually actively involved in the catalytic reaction of the enzyme.
B) They tend to be stable to heat.
C) They can serve as intermediate carriers of functional groups.
D) They are protein components.
E) They may contain vitamins as part of their structure.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements is true regarding enzyme pathways?
A) the most effective way to control a pathway is to regulate every enzyme in the pathway
B) an enzyme pathway always proceeds in only one direction, never in reverse
C) a regulatory enzyme is regulated only by molecules within the given pathway
D) metabolic pathways are necessary since enzymes usually catalyze only one specific reaction
E) none of the above are true
A) the most effective way to control a pathway is to regulate every enzyme in the pathway
B) an enzyme pathway always proceeds in only one direction, never in reverse
C) a regulatory enzyme is regulated only by molecules within the given pathway
D) metabolic pathways are necessary since enzymes usually catalyze only one specific reaction
E) none of the above are true

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
10
Enzymes work by:
A) providing an alternate reaction pathway
B) increasing the spontaneity (ΔG) of the reaction
C) lowering the activation energy of the reaction
D) altering the concentration of the reactants to achieve a favorable ΔG
E) proceeding in only one direction, from reactants to products
A) providing an alternate reaction pathway
B) increasing the spontaneity (ΔG) of the reaction
C) lowering the activation energy of the reaction
D) altering the concentration of the reactants to achieve a favorable ΔG
E) proceeding in only one direction, from reactants to products

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
11
For an enzyme-catalyzed reaction, the initial velocity was determined at two different concentrations of the substrate. Which of the following would be closest to the value of Vmax?
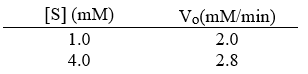
A) 4.7 mM/min
B) 0.67 mM/min
C) 3.19 mM/min
D) 1.5 mM/min
E) 0.32 mM/min
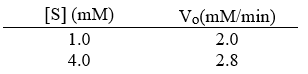
A) 4.7 mM/min
B) 0.67 mM/min
C) 3.19 mM/min
D) 1.5 mM/min
E) 0.32 mM/min

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
12
Which statement is correct about the Michaelis-Menten constant, Km, for the kinetic mechanism below? 
A) It is numerically equal to the substrate concentration required to achieve one half the maximum velocity.
B) Its defined as Km = k1/(k−1 + k2).
C) It is approximately equal to the dissociation constant for the enzyme-substrate complex to E + P.
D) The value of Km is constant for an enzyme regardless of the specific substrate molecule used to determine it.
E) Its numeric value has the units of moles−1.

A) It is numerically equal to the substrate concentration required to achieve one half the maximum velocity.
B) Its defined as Km = k1/(k−1 + k2).
C) It is approximately equal to the dissociation constant for the enzyme-substrate complex to E + P.
D) The value of Km is constant for an enzyme regardless of the specific substrate molecule used to determine it.
E) Its numeric value has the units of moles−1.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
13
If the rate constant for the enzyme catalyzed reaction is 2 × 105/sec and the rate constant for the uncatalyzed reaction is 2 × 10−6/sec, the catalytic power of the enzyme is:
A) 1011
B) 2 × 10−11
C) 10−11
D) 10−1
E) 2 × 10−1
A) 1011
B) 2 × 10−11
C) 10−11
D) 10−1
E) 2 × 10−1

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
14
An enzyme's specificity can be due to:
A) the ratio of catalyzed rate to the uncatalyzed rate of reaction.
B) molecular recognition based on structural complementarity.
C) amount of enzyme produced by the cell.
D) amount of substrate available.
E) metabolic activators.
A) the ratio of catalyzed rate to the uncatalyzed rate of reaction.
B) molecular recognition based on structural complementarity.
C) amount of enzyme produced by the cell.
D) amount of substrate available.
E) metabolic activators.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
15
The catalytically active complex of an apoenzyme and its prosthetic group is referred to a(n) ____.
A) catalytic duo
B) holoenzyme
C) prosthetic enzyme
D) dimeric enzyme
E) none of the above
A) catalytic duo
B) holoenzyme
C) prosthetic enzyme
D) dimeric enzyme
E) none of the above

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
16
All of the following statements are true about the relationships between [S], Km and Vmax EXCEPT:
A) As the [S] is increased, v approaches the limiting value, Vmax.
B) Km = Vmax/2.
C) The rate of the reaction, v, follows a first order rate equation v = K'[A] and K' = Vmax/Km.
D) The rate of product formed, v, is at Vmax when [S] >> Km.
E) Km and Vmax assist in finding the rate of the enzyme catalyzed reaction only if the reaction is irreversible.
A) As the [S] is increased, v approaches the limiting value, Vmax.
B) Km = Vmax/2.
C) The rate of the reaction, v, follows a first order rate equation v = K'[A] and K' = Vmax/Km.
D) The rate of product formed, v, is at Vmax when [S] >> Km.
E) Km and Vmax assist in finding the rate of the enzyme catalyzed reaction only if the reaction is irreversible.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
17
For an enzyme-catalyzed reaction, the initial velocity was determined at two different concentrations of the substrate. Which of the following would be closest to the value of Km?
[S] (mM)
Vo(mM/min)
1)0
2)0
4)0
2)8
A) 0.17 mM
B) 5.7 mM
C) 2.7 mM
D) 0.60 mM
E) 1.7 mM
[S] (mM)
Vo(mM/min)
1)0
2)0
4)0
2)8
A) 0.17 mM
B) 5.7 mM
C) 2.7 mM
D) 0.60 mM
E) 1.7 mM

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
18
How do catalysts work to accelerate a chemical reaction?
A) They raise the average energy of the reactants.
B) They provide a means of acceleration by being completely consumed in the reaction.
C) They lower the energy of activation.
D) They lower the overall free energy change of the reaction.
E) They raise the overall free energy change of the reaction.
A) They raise the average energy of the reactants.
B) They provide a means of acceleration by being completely consumed in the reaction.
C) They lower the energy of activation.
D) They lower the overall free energy change of the reaction.
E) They raise the overall free energy change of the reaction.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
19
If an enzyme has a Vmax of 15 mM/min, what is the velocity if the substrate is present at 1/5 of the Km?
A) 12 mM/min
B) 6 mM/min
C) 3 mM/min
D) 2.5 mM/min
E) cannot be determined from given information
A) 12 mM/min
B) 6 mM/min
C) 3 mM/min
D) 2.5 mM/min
E) cannot be determined from given information

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
20
When every enzyme molecule in the reaction mixture has its substrate-binding site occupied by substrate, the kinetics become ____-order, and the velocity is ____.
A) zero; Vmax
B) first; Vmax
C) second; Vmax/2
D) zero; Vmax/2
E) first; Vmax/2
A) zero; Vmax
B) first; Vmax
C) second; Vmax/2
D) zero; Vmax/2
E) first; Vmax/2

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
21
All of the following statements about competitive inhibition are correct EXCEPT:
A) Competitive inhibitors are often chemical analogs of the substrate.
B) For a group-specific enzyme, one substrate would be a competitive inhibitor of reactions of the other possible substrate.
C) Sometimes a product of an enzyme-catalyzed reaction is a competitive inhibitor of its own production.
D) In the presence of a competitive inhibitor, the apparent Km would be altered and Vmax would be decreased.
E) Competitive inhibitors usually interact with the enzyme at the binding site for a substrate.
A) Competitive inhibitors are often chemical analogs of the substrate.
B) For a group-specific enzyme, one substrate would be a competitive inhibitor of reactions of the other possible substrate.
C) Sometimes a product of an enzyme-catalyzed reaction is a competitive inhibitor of its own production.
D) In the presence of a competitive inhibitor, the apparent Km would be altered and Vmax would be decreased.
E) Competitive inhibitors usually interact with the enzyme at the binding site for a substrate.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
22
All are characteristics of ribozymes EXCEPT:
A) They are critical for removal of introns from mRNA
B) They are substrate specific.
C) They enhance the reaction rate.
D) On the ribosome, they catalyze the peptidyl transferase reaction
E) All are true.
A) They are critical for removal of introns from mRNA
B) They are substrate specific.
C) They enhance the reaction rate.
D) On the ribosome, they catalyze the peptidyl transferase reaction
E) All are true.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
23
All are characteristics of ordered single-displacement reactions EXCEPT:
A) Lineweaver-Burk plots with lines that intersect to the left of the 1/v axis.
B) a chemically modified enzyme-intermediate.
C) the lack of any exchange reaction activity.
D) the lack of competitive substrate effects.
E) single substrate initial binding activity.
A) Lineweaver-Burk plots with lines that intersect to the left of the 1/v axis.
B) a chemically modified enzyme-intermediate.
C) the lack of any exchange reaction activity.
D) the lack of competitive substrate effects.
E) single substrate initial binding activity.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
24
Sildenafil citrate (Viagra) was developed as an inhibitor of:
A) acid phosphatases.
B) cAMP phosphodiesterases.
C) glycogen phosphorylase.
D) cGMP phosphodiesterases.
E) testosterone reductase.
A) acid phosphatases.
B) cAMP phosphodiesterases.
C) glycogen phosphorylase.
D) cGMP phosphodiesterases.
E) testosterone reductase.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
25
A plot of 1/V vs. 1/[S] for an enzyme catalyzed reaction gave a line with an equation of y = 0.5x + 0.2. The same enzyme with an inhibitor present gave a line with an equation of y = 1.1x + 0.2. Which of the following statements is true?
A) the type of inhibition is competitive
B) the type of inhibition is noncompetitive
C) the type of inhibition is uncompetitive
D) the Vmax with the inhibitor present has decreased
E) the Km with the inhibitor present has decreased
A) the type of inhibition is competitive
B) the type of inhibition is noncompetitive
C) the type of inhibition is uncompetitive
D) the Vmax with the inhibitor present has decreased
E) the Km with the inhibitor present has decreased

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
26
In the enzyme catalyzed reaction sequence below, can the E-PO4− intermediate be predicted and why? 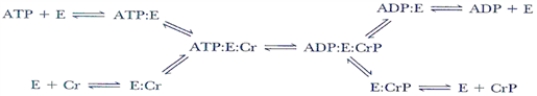
A) Yes, the mechanism is a double-displacement reaction.
B) Yes, the reaction fits the ping-pong model.
C) No, the reaction is random single-displacement.
D) No, the reaction is double-displacement.
E) None of the above.
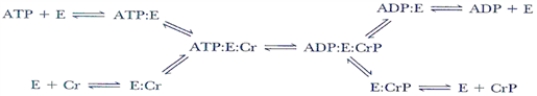
A) Yes, the mechanism is a double-displacement reaction.
B) Yes, the reaction fits the ping-pong model.
C) No, the reaction is random single-displacement.
D) No, the reaction is double-displacement.
E) None of the above.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
27
All are true for kcat EXCEPT :
A) referred to as the molecular activity of the enzyme.
B) called the turnover number of the enzyme.
C) measures the maximal catalytic activity or kinetic efficiency of an enzyme.
D) defines the number of substrate molecules converted into product/enzyme molecule/unit of time when the enzyme is saturated with substrate.
E) all are true.
A) referred to as the molecular activity of the enzyme.
B) called the turnover number of the enzyme.
C) measures the maximal catalytic activity or kinetic efficiency of an enzyme.
D) defines the number of substrate molecules converted into product/enzyme molecule/unit of time when the enzyme is saturated with substrate.
E) all are true.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
28
Penicillin and other β-lactam antibiotics form a covalent bond to the enzyme glycoprotein transpeptidase. What type of bond is formed?
A) ester
B) thioester
C) amide
D) phosphate
E) none of the above
A) ester
B) thioester
C) amide
D) phosphate
E) none of the above

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
29
Malonate inhibition of succinate dehydrogenase is an example of:
A) noncompetitive inhibition.
B) competitive inhibition.
C) mixed noncompetitive inhibition.
D) irreversible inhibition.
E) uncompetitive inhibition.
A) noncompetitive inhibition.
B) competitive inhibition.
C) mixed noncompetitive inhibition.
D) irreversible inhibition.
E) uncompetitive inhibition.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following statements is NOT characteristic of kcat/Km?
A) It corresponds to a second-order rate constant.
B) It provides an excellent parameter for comparison of the catalytic efficiency of enzymes.
C) It reflects the property of the enzyme when substrate concentration is at saturation.
D) The upper limit for the kcat/Km value is fixed by the diffusion-controlled limit for reactions, which is 109 M−1 s−1.
E) It is also referred to as the turnover number.
A) It corresponds to a second-order rate constant.
B) It provides an excellent parameter for comparison of the catalytic efficiency of enzymes.
C) It reflects the property of the enzyme when substrate concentration is at saturation.
D) The upper limit for the kcat/Km value is fixed by the diffusion-controlled limit for reactions, which is 109 M−1 s−1.
E) It is also referred to as the turnover number.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
31
Catalytic antibodies, also called ____, are generated against an antigen that is:
A) abzymes; an analog of the transition-state intermediate in the reaction.
B) abzymes; the substrate of the reaction.
C) zymogens; an analog of the product of the reaction.
D) holoenzyme; an analog of the transition-state intermediate in the reaction.
E) none of the above.
A) abzymes; an analog of the transition-state intermediate in the reaction.
B) abzymes; the substrate of the reaction.
C) zymogens; an analog of the product of the reaction.
D) holoenzyme; an analog of the transition-state intermediate in the reaction.
E) none of the above.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
32
In transforming the Michaelis-Menten equation into a straight line equation, y = mx + b, the Lineweaver-Burk double reciprocal plot, which of the following is NOT a true representation?
A) slope = Km/Vmax
B) y-intercept is 1/Vmax
C) x-intercept is 1/Km
D) y = 1/V
E) x = 1/[S]
A) slope = Km/Vmax
B) y-intercept is 1/Vmax
C) x-intercept is 1/Km
D) y = 1/V
E) x = 1/[S]

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
33
Identify the type of reaction that would give the following graph: 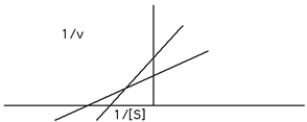
A) double displacement bisubstrate reaction.
B) competitively inhibited reaction.
C) single displacement bisubstrate reaction.
D) mixed noncompetitively inhibited reaction.
E) pure noncompetitively inhibited reaction.

A) double displacement bisubstrate reaction.
B) competitively inhibited reaction.
C) single displacement bisubstrate reaction.
D) mixed noncompetitively inhibited reaction.
E) pure noncompetitively inhibited reaction.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
34
In uncompetitive inhibition:
A) I combines only with ES.
B) I combines only with E.
C) I combines with E and ES.
D) I combines with EP.
E) none of the above.
A) I combines only with ES.
B) I combines only with E.
C) I combines with E and ES.
D) I combines with EP.
E) none of the above.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
35
All of the following statements about noncompetitive inhibition are true EXCEPT:
A) They interact with the enzyme as well as the enzyme-substrate complex.
B) Increasing the concentration of [S] can overcome the inhibition.
C) The Vmax value does not remain the same as for a reaction that is not inhibited.
D) The inhibitor can cause a conformational change in the enzyme.
E) The inhibitor binds to a different site than does the substrate.
A) They interact with the enzyme as well as the enzyme-substrate complex.
B) Increasing the concentration of [S] can overcome the inhibition.
C) The Vmax value does not remain the same as for a reaction that is not inhibited.
D) The inhibitor can cause a conformational change in the enzyme.
E) The inhibitor binds to a different site than does the substrate.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
36
The International Units of an enzyme are based on the:
A) ratio of enzyme to other proteins.
B) micromoles of product formed per minute.
C) moles of substrate reacted.
D) micromoles of product produced at Vmax/2.
E) none of the above.
A) ratio of enzyme to other proteins.
B) micromoles of product formed per minute.
C) moles of substrate reacted.
D) micromoles of product produced at Vmax/2.
E) none of the above.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
37
Penicillin is an example of a mechanism-based enzyme inactivator and is a(n):
A) competitive inhibitor.
B) noncompetitive inhibitor.
C) suicide substrate.
D) uncompetitive inhibitor.
E) none of the above.
A) competitive inhibitor.
B) noncompetitive inhibitor.
C) suicide substrate.
D) uncompetitive inhibitor.
E) none of the above.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
38
In the glutamate:aspartate aminotransferase catalyzed reaction mechanism, glutamate would react with ____ to form α-ketoglutarate.
A) E-pyridoxal phosphate complex
B) E-pyridoxamine phosphate complex
C) aspartate
D) oxaloacetate
E) none of the above
A) E-pyridoxal phosphate complex
B) E-pyridoxamine phosphate complex
C) aspartate
D) oxaloacetate
E) none of the above

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
39
All are true for inhibitor I if it is a competitive inhibitor EXCEPT:
A) It binds a site other than the active site.
B) It is structurally similar to the substrate.
C) EI does not give rise to E + P.
D) For a given [I], v decreases.
E) At some point, S can displace all of I on E.
A) It binds a site other than the active site.
B) It is structurally similar to the substrate.
C) EI does not give rise to E + P.
D) For a given [I], v decreases.
E) At some point, S can displace all of I on E.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
40
In the reaction mechanism below, ____ are competitive for binding to free enzyme E. 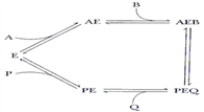
A) P and A
B) A and B
C) B and Q
D) Q and A
E) All of the above

A) P and A
B) A and B
C) B and Q
D) Q and A
E) All of the above

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
41
Explain bimolecular reactions.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
42
Discuss the effect of substrate concentration, [S], on the rate of a reaction, v.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
43
Explain the mechanism of action of penicillin as an example of irreversible inhibition.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
44
Discuss the induced fit model that explains enzyme specificity.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
45
All are characteristics of the enzyme hexokinase EXCEPT:
A) It is not highly specific and will catalyze phosphorylation of a number of hexoses at the six position.
B) Hexoses bind the active site and induce a solvent inaccessible fit to the hexose.
C) Glycerol may fit into the active site, but it does not bind to induce a conformational change necessary for catalysis.
D) When a hexose binds the active site, the active site is modified to promote changes in the substrate to a transitional-state intermediate.
E) All are true.
A) It is not highly specific and will catalyze phosphorylation of a number of hexoses at the six position.
B) Hexoses bind the active site and induce a solvent inaccessible fit to the hexose.
C) Glycerol may fit into the active site, but it does not bind to induce a conformational change necessary for catalysis.
D) When a hexose binds the active site, the active site is modified to promote changes in the substrate to a transitional-state intermediate.
E) All are true.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following statements regarding inhibitors is correct?
A) a transition state analog binds covalently to the enzyme
B) a non-competitive inhibitor causes an increase in the Vmax
C) the presence of a competitive inhibitor can be overcome by addition of more inhibitor
D) a competitive inhibitor results in a higher apparent Km value
E) none of the above are correct
A) a transition state analog binds covalently to the enzyme
B) a non-competitive inhibitor causes an increase in the Vmax
C) the presence of a competitive inhibitor can be overcome by addition of more inhibitor
D) a competitive inhibitor results in a higher apparent Km value
E) none of the above are correct

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
47
How is a Lineweaver-Burk plot obtained?

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
48
Carbonic anhydrase has two substrates, carbon dioxide and bicarbonate, which are both converted to carbonic acid. Kinetic data for each is given below. While determining the kinetics of HCO3- as a substrate, how would the addition of CO2 effect the reaction if the rate were measured by the disappearance of bicarbonate?
Substrate
Km (mM)
Kcat (sec-1)
Kcat/Km (m M-1sec-1)
CO2
HCO3-
12
26
1×106
4×105
8)3×104
1.5×104
A) CO2 would increase the activity of the enzyme
B) CO2 would cause an apparent decrease in the Km for HCO3-
C) CO2 would act as a noncompetitive inhibitor
D) CO2 would act as a competitive inhibitor
E) none of the above
Substrate
Km (mM)
Kcat (sec-1)
Kcat/Km (m M-1sec-1)
CO2
HCO3-
12
26
1×106
4×105
8)3×104
1.5×104
A) CO2 would increase the activity of the enzyme
B) CO2 would cause an apparent decrease in the Km for HCO3-
C) CO2 would act as a noncompetitive inhibitor
D) CO2 would act as a competitive inhibitor
E) none of the above

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
49
An enzymatic reaction has a Vmax of 30 μM/min and a Km of 50 μM. If the concentration of the substrate is 25 μM, which of the following is true?
A) the velocity will be 7.5 μM/min
B) the velocity will be 20 μM/min
C) the velocity will be 10 μM/min
D) the velocity will be 25 μM/min
E) the velocity will be 15 μM/min
A) the velocity will be 7.5 μM/min
B) the velocity will be 20 μM/min
C) the velocity will be 10 μM/min
D) the velocity will be 25 μM/min
E) the velocity will be 15 μM/min

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
50
Carbonic anhydrase has two substrates, carbon dioxide and bicarbonate, which are both converted to carbonic acid. Kinetic data for each is given below. Typical values for Kcat/Km range from less than 1 mM-1sec-1 to as high as 2.8×105 mM-1sec-1. What can be said about the Kcat/Km for CO2?
Substrate
Km (mM)
Kcat (sec-1)
Kcat/Km (m M-1sec-1)
CO2
HCO3-
12
26
1×106
4×105
8)3×104
1.5×104
A) carbonic anhydrase binds CO2 well but poorly converts it to carbonic acid
B) carbonic anhydrase binds CO2 poorly but effectively converts it to carbonic acid
C) carbonic anhydrase binds CO2 well and converts it to carbonic acid very well
D) carbonic anhydrase binds CO2 poorly and poorly converts it to carbonic acid
E) the data above is useless for this question
Substrate
Km (mM)
Kcat (sec-1)
Kcat/Km (m M-1sec-1)
CO2
HCO3-
12
26
1×106
4×105
8)3×104
1.5×104
A) carbonic anhydrase binds CO2 well but poorly converts it to carbonic acid
B) carbonic anhydrase binds CO2 poorly but effectively converts it to carbonic acid
C) carbonic anhydrase binds CO2 well and converts it to carbonic acid very well
D) carbonic anhydrase binds CO2 poorly and poorly converts it to carbonic acid
E) the data above is useless for this question

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck


