Exam 13: Enzymeskinetics and Specificity
Exam 1: The Facts of Life: Chemistry Is the Logic of Biological Phenomena36 Questions
Exam 2: Water: the Medium of Life43 Questions
Exam 3: Thermodynamics of Biological Systems41 Questions
Exam 4: Amino Acids and the Peptide Bond29 Questions
Exam 5: Proteins: Their Primary Structure and Biological Functions47 Questions
Exam 6: Proteins: Secondary, Tertiary, and Quaternary Structure58 Questions
Exam 7: Carbohydrates and the Glycoconjugates of Cell Surfaces58 Questions
Exam 8: Lipids35 Questions
Exam 9: Membranes and Membrane Transport44 Questions
Exam 10: Nucleotides and Nucleic Acids39 Questions
Exam 11: Structure of Nucleic Acids35 Questions
Exam 12: Recombinant Dna, Cloning, Chimeric Genes, and Synthetic Biology37 Questions
Exam 13: Enzymeskinetics and Specificity50 Questions
Exam 14: Mechanisms of Enzyme Action34 Questions
Exam 15: Enzyme Regulation40 Questions
Exam 16: Molecular Motors35 Questions
Exam 17: Metabolism: an Overview68 Questions
Exam 18: Glycolysis67 Questions
Exam 19: The Tricarboxylic Acid Cycle56 Questions
Exam 20: Electron Transport and Oxidative Phosphorylation62 Questions
Exam 21: Photosynthesis62 Questions
Exam 22: Gluconeogenesis, Glycogen Metabolism, and the Pentose Phosphate Pathway60 Questions
Exam 23: Fatty Acid Catabolism41 Questions
Exam 24: Lipid Biosynthesis70 Questions
Exam 25: Nitrogen Acquisition and Amino Acid Metabolism55 Questions
Exam 26: Synthesis and Degradation of Nucleotides41 Questions
Exam 27: Metabolic Integration and Organ Specialization47 Questions
Exam 28: Dna Metabolism: Replication, Recombination, and Repair68 Questions
Exam 29: Transcription and the Regulation of Gene Expression68 Questions
Exam 30: Protein Synthesis58 Questions
Exam 31: Completing the Protein Life Cycle: Folding, Processing, and Degradation36 Questions
Exam 32: The Reception and Transmission of Extracellular Information58 Questions
Select questions type
An enzymatic reaction has a Vmax of 30 μM/min and a Km of 50 μM. If the concentration of the substrate is 25 μM, which of the following is true?
Free
(Multiple Choice)
4.8/5  (42)
(42)
Correct Answer:
B
Which of the following is true regarding the Briggs and Haldane steady state assumption?
Free
(Multiple Choice)
4.8/5  (29)
(29)
Correct Answer:
C
Carbonic anhydrase has two substrates, carbon dioxide and bicarbonate, which are both converted to carbonic acid. Kinetic data for each is given below. While determining the kinetics of HCO3- as a substrate, how would the addition of CO2 effect the reaction if the rate were measured by the disappearance of bicarbonate?
Substrate
Km (mM)
Kcat (sec-1)
Kcat/Km (m M-1sec-1)
CO2
HCO3-
12
26
1×106
4×105
8)3×104
1.5×104
Free
(Multiple Choice)
4.8/5  (32)
(32)
Correct Answer:
D
All are true for inhibitor I if it is a competitive inhibitor EXCEPT:
(Multiple Choice)
4.9/5  (35)
(35)
Discuss the effect of substrate concentration, [S], on the rate of a reaction, v.
(Essay)
4.9/5  (28)
(28)
In transforming the Michaelis-Menten equation into a straight line equation, y = mx + b, the Lineweaver-Burk double reciprocal plot, which of the following is NOT a true representation?
(Multiple Choice)
5.0/5  (33)
(33)
Penicillin and other β-lactam antibiotics form a covalent bond to the enzyme glycoprotein transpeptidase. What type of bond is formed?
(Multiple Choice)
4.8/5  (29)
(29)
For an enzyme-catalyzed reaction, the initial velocity was determined at two different concentrations of the substrate. Which of the following would be closest to the value of Km?
[S] (mM)
Vo(mM/min)
1)0
2)0
4)0
2)8
(Multiple Choice)
4.9/5  (27)
(27)
The specific site on the enzyme where ____ binds and catalysis occurs is called the ____ site.
(Multiple Choice)
4.8/5  (39)
(39)
If the rate constant for the enzyme catalyzed reaction is 2 × 105/sec and the rate constant for the uncatalyzed reaction is 2 × 10−6/sec, the catalytic power of the enzyme is:
(Multiple Choice)
4.9/5  (35)
(35)
A plot of 1/V vs. 1/[S] for an enzyme catalyzed reaction gave a line with an equation of y = 0.5x + 0.2. The same enzyme with an inhibitor present gave a line with an equation of y = 1.1x + 0.2. Which of the following statements is true?
(Multiple Choice)
4.9/5  (31)
(31)
In the enzyme catalyzed reaction sequence below, can the E-PO4− intermediate be predicted and why? 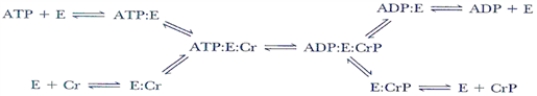
(Multiple Choice)
4.9/5  (37)
(37)
Showing 1 - 20 of 50
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)