Deck 7: Solutions and Colloids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/87
Play
Full screen (f)
Deck 7: Solutions and Colloids
1
What volume of a 10.00% (w/v)solution of sugar is needed to provide 2.00 g of sugar?
A)0.200 liter
B)1.00 mL
C)20.0 mL
D)5.00 mL
A)0.200 liter
B)1.00 mL
C)20.0 mL
D)5.00 mL
20.0 mL
2
How many moles of Na2CO3 are needed to react with 750 mL of 0.250 M H2SO4 solution? Na2CO3 + H2SO4 Na2SO4 + CO2 + H2O
A)3.00* 103
B)0.333
C)1.33
D)0.188
A)3.00* 103
B)0.333
C)1.33
D)0.188
0.188
3
As NH4NO3 dissolves in water,the resulting solution becomes colder.Which of the following expressions is most correct?
A)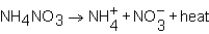
B)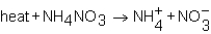
C)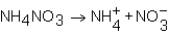
D)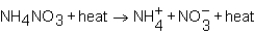
A)
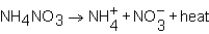
B)
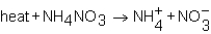
C)
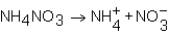
D)
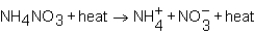
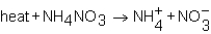
4
A solution is made by dissolving 5.84 grams of NaCl in enough distilled water to give a final volume of 1.00 L.What is the molarity of the solution?
A)0.100
B)1.00
C)0.0250
D)0.400
A)0.100
B)1.00
C)0.0250
D)0.400

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following statements relating to a solution is not correct?
A)A solution may contain more than one solute.
B)A solution may contain only one solute.
C)Water is always the solvent in a solution.
D)More than one correct response given.
A)A solution may contain more than one solute.
B)A solution may contain only one solute.
C)Water is always the solvent in a solution.
D)More than one correct response given.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
6
A solution is made by dissolving 15.0 mL of oil in enough gasoline to give 50.0 mL of solution.What is the % (v/v)of oil in the solution?
A)30.0
B)23.1
C)42.9
D)3.33
A)30.0
B)23.1
C)42.9
D)3.33

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
7
You want to remove as much CO2 gas as possible from a water solution.Which of the following treatments would be most effective?
A)cool the solution
B)filter the solution
C)boil the solution
D)aerate the solution
A)cool the solution
B)filter the solution
C)boil the solution
D)aerate the solution

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
8
A solution is made by dissolving a small amount of salt in a beaker of water.The water is referred to as the
A)precipitate.
B)filtrate.
C)solvent.
D)solute.
A)precipitate.
B)filtrate.
C)solvent.
D)solute.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
9
How many mL of 6.00 M HCl are needed to prepare 1500 mL of 0.200 M HCl solution?
A)1.80 *104
B)125
C)2.00 *10-3
D)50.0
A)1.80 *104
B)125
C)2.00 *10-3
D)50.0

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following pairs can produce a homogeneous mixture?
A)water and sand
B)oil and vinegar
C)vinegar and glucose
D)calcium carbonate and water
A)water and sand
B)oil and vinegar
C)vinegar and glucose
D)calcium carbonate and water

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
11
How many grams of solid KCl are needed to prepare 250 mL of 0.235 M solution?
A)9.32
B)31.3
C)15.6
D)4.38
A)9.32
B)31.3
C)15.6
D)4.38

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
12
When solid NaOH is dissolved in water,the solution becomes hot.The solution process is
A)exothermic.
B)endothermic.
C)neither exothermic nor endothermic.
D)Can't be classified.
A)exothermic.
B)endothermic.
C)neither exothermic nor endothermic.
D)Can't be classified.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
13
Suppose a solution contains 200 g water and 15 g glucose.Which of the following statements is true?
A)Glucose would be classified as the solvent.
B)Water would be classified as the solvent.
C)Either component could be classified as the solvent.
D)More than one response is correct.
A)Glucose would be classified as the solvent.
B)Water would be classified as the solvent.
C)Either component could be classified as the solvent.
D)More than one response is correct.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
14
What is the molarity of a solution containing 0.325 moles of solute in 250 mL of solution?
A)1.30 *10-3
B)1.30
C)0.769
D)8.13 * 10-2
A)1.30 *10-3
B)1.30
C)0.769
D)8.13 * 10-2

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
15
Iodine,I2,is very slightly soluble in water,a polar solvent,but quite soluble in toluene,a nonpolar solvent.What can be inferred about the nature of the I2 molecule?
A)It is ionic.
B)It is polar.
C)It is nonpolar.
D)Nothing can be inferred.
A)It is ionic.
B)It is polar.
C)It is nonpolar.
D)Nothing can be inferred.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
16
A salt sample is placed into some water and nearly all of it dissolves without stirring or heating.The resulting solution is
A)saturated.
B)supersaturated.
C)unsaturated.
D)impossible to determine.
A)saturated.
B)supersaturated.
C)unsaturated.
D)impossible to determine.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
17
When will carbon dioxide in a carbonated soft drink dissolve best?
A)after shaking the container
B)after heating the container
C)after cooling the container
D)after releasing the pressure
A)after shaking the container
B)after heating the container
C)after cooling the container
D)after releasing the pressure

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
18
A solution is made by combining 4.00 g of sugar and 100 mL of water (density = 1.00 g/mL).What is the concentration in % w/w?
A)26.0
B)4.00
C)0.0400
D)3.85
A)26.0
B)4.00
C)0.0400
D)3.85

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
19
A solution is produced in which water is the solvent and there are four solutes.Which of the solutes can dissolve better if the solution is heated?
A)oxygen
B)sodium bicarbonate
C)argon
D)more than one response is right
A)oxygen
B)sodium bicarbonate
C)argon
D)more than one response is right

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
20
Which compound is most soluble in a polar solvent?
A)silver nitrate
B)silver chloride
C)silver carbonate
D)all are of equal solubility
A)silver nitrate
B)silver chloride
C)silver carbonate
D)all are of equal solubility

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
21
Compared to pure water,a salt water solution will have a
A)lower vapor pressure,freezing point and boiling point.
B)higher vapor pressure,freezing point and boiling point.
C)lower vapor pressure and freezing point and a higher boiling point.
D)higher freezing point and a lower vapor pressure and boiling point.
A)lower vapor pressure,freezing point and boiling point.
B)higher vapor pressure,freezing point and boiling point.
C)lower vapor pressure and freezing point and a higher boiling point.
D)higher freezing point and a lower vapor pressure and boiling point.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
22
A solution is prepared by dissolving 4.66 g of KCl in enough distilled water to give 250 mL of solution.KCl is a strong electrolyte.How will the freezing point of the solution be different from that of pure water? (Note: Kf for water is 1.86°C/M.)
A)solution will be 0.930°C lower than water
B)solution will be 0.475°C lower than pure water
C)solution will be 0.930°C higher than pure water
D)solution will be 0.475°C higher than pure water
A)solution will be 0.930°C lower than water
B)solution will be 0.475°C lower than pure water
C)solution will be 0.930°C higher than pure water
D)solution will be 0.475°C higher than pure water

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
23
A solution is prepared by adding 25.0 mL of 1.30 M AlCl3 solution to a flask,and then adding enough water to give a final volume of 200.0 mL.What is the molarity of the solution?
A)0.260
B)0.163
C)6.50
D)1.24
A)0.260
B)0.163
C)6.50
D)1.24

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
24
Calculate the number of moles of ZnCl,in 100 mL of 0.300 M solution.
A)3.00* 10-2
B)0.300
C)30.0
D)3.00
A)3.00* 10-2
B)0.300
C)30.0
D)3.00

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
25
The rate of osmosis
A)can be increased by having larger pores in the membrane.
B)can be increased by removing the membrane.
C)can be increased by an increase in the atmospheric pressure.
D)cannot be increased by any of the responses.
A)can be increased by having larger pores in the membrane.
B)can be increased by removing the membrane.
C)can be increased by an increase in the atmospheric pressure.
D)cannot be increased by any of the responses.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
26
A 0.9% NaCl (w/w)solution in water is
A)produced by mixing 0.9 moles of NaCl in a 100 moles of water.
B)produced and has the same final volume as a 0.9% solution in ethyl alcohol.
C)a solution that boils at or above 100 C.
D)All of these responses are correct.
A)produced by mixing 0.9 moles of NaCl in a 100 moles of water.
B)produced and has the same final volume as a 0.9% solution in ethyl alcohol.
C)a solution that boils at or above 100 C.
D)All of these responses are correct.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following tend to stabilize colloids and prevent suspended particles from settling?
A)presence of emulsifying agents
B)absorption of charges by colloid particles
C)absence of ionic salts
D)more than one response is correct
A)presence of emulsifying agents
B)absorption of charges by colloid particles
C)absence of ionic salts
D)more than one response is correct

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
28
Changes in boiling point,freezing point,and vapor pressure are
A)the same for 2 M NaCl as they are for 2 M CaCl2.
B)the same for 50 mL of 2 M NaCl as they are for 250 mL of 1 M NaCl.
C)the same for 75 mL of 2 M NaCl as they are for 50 mL of 2 M NaOH.
D)the same at 1 atm as at 1.55 atm.
A)the same for 2 M NaCl as they are for 2 M CaCl2.
B)the same for 50 mL of 2 M NaCl as they are for 250 mL of 1 M NaCl.
C)the same for 75 mL of 2 M NaCl as they are for 50 mL of 2 M NaOH.
D)the same at 1 atm as at 1.55 atm.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following pass through both osmotic and dialysis membranes?
A)solvent molecules
B)large molecules
C)cations
D)anions
A)solvent molecules
B)large molecules
C)cations
D)anions

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
30
An isotonic salt solution is 0.90% (w/w)NaCl in water.How many grams of NaCl are contained in 1.00 kg of such a solution?
A)0.090
B)0.90
C)9.0
D)90
A)0.090
B)0.90
C)9.0
D)90

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following pairs correctly represent similar functions for a solution component and a colloid component?
A)solvent/dispersed phase
B)solute/dispersion medium
C)solvent/dispersion medium
D)more than one response is correct
A)solvent/dispersed phase
B)solute/dispersion medium
C)solvent/dispersion medium
D)more than one response is correct

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
32
The boiling point of a solution of sugar water is
A)higher than that of pure solvent.
B)the same as that of pure solvent.
C)lower than that of pure solvent.
D)impossible to determine.
A)higher than that of pure solvent.
B)the same as that of pure solvent.
C)lower than that of pure solvent.
D)impossible to determine.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
33
Two solutions with concentrations of 2% sugar and 4% sugar,respectively,are separated by a semipermeable membrane.During osmosis,there is a net flow of
A)sugar molecules from the concentrated to the dilute solution.
B)sugar molecules from the dilute to the concentrated solution.
C)water molecules from the concentrated to the dilute solution.
D)water molecules from the dilute to the concentrated solution.
A)sugar molecules from the concentrated to the dilute solution.
B)sugar molecules from the dilute to the concentrated solution.
C)water molecules from the concentrated to the dilute solution.
D)water molecules from the dilute to the concentrated solution.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
34
In the colloid known as mayonnaise,the dispersed phase is _____ .
A)a gas
B)a liquid
C)a solid
D)unknown
A)a gas
B)a liquid
C)a solid
D)unknown

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
35
The spores from many molds produce an allergic reaction in many individuals.If mold spores in the air were to be considered a colloid,which technique would remove the spores best from a functioning air conditioner duct?
A)Place a filter made from woven fiberglass fibers in the duct.
B)Install an electrical device with both positive and negative poles.
C)Install an ultraviolet light producing device in the duct.
D)All of these responses work to the same extent.
A)Place a filter made from woven fiberglass fibers in the duct.
B)Install an electrical device with both positive and negative poles.
C)Install an ultraviolet light producing device in the duct.
D)All of these responses work to the same extent.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
36
What is the underlying process involved in dialysis?
A)mixtures are homogenous.
B)osmosis is due to a difference in solution concentrations.
C)pressure is applied to the blood squeezing out the impurities.
D)an electrical potential separates the blood from the impurities.
A)mixtures are homogenous.
B)osmosis is due to a difference in solution concentrations.
C)pressure is applied to the blood squeezing out the impurities.
D)an electrical potential separates the blood from the impurities.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
37
What is the osmotic pressure of a 0.050 M solution of AlCl3 in water that is at 0.00 C? Consider AlCl3 to be a strong electrolyte.
A)8.5 *102 torr
B)1.1 atm
C)2.2 atm
D)3.4 * 103 torr
A)8.5 *102 torr
B)1.1 atm
C)2.2 atm
D)3.4 * 103 torr

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
38
The vapor pressure of a pure solvent is
A)higher than that of a solution.
B)impossible to determine.
C)the same as that of a solution.
D)lower than that of a solution.
A)higher than that of a solution.
B)impossible to determine.
C)the same as that of a solution.
D)lower than that of a solution.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
39
What volume of ethyl alcohol is contained in 35 mL (1 oz.)of 86 proof liquor,which is 43% (v/v)alcohol?
A)30 mL
B)43 mL
C)15 mL
D)37 mL
A)30 mL
B)43 mL
C)15 mL
D)37 mL

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
40
You have a patient who is suffering from the "bends".What gas is in excess in the blood?
A)nitrogen
B)oxygen
C)helium
D)all of these
A)nitrogen
B)oxygen
C)helium
D)all of these

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
41
The ability to see the scattering of light when passed through a colloid is known as
A)the dispersing effect.
B)a scattering ratio.
C)an emulsifying agent.
D)the Tyndall effect.
A)the dispersing effect.
B)a scattering ratio.
C)an emulsifying agent.
D)the Tyndall effect.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
42
The primary intermolecular attractions between CH3-OH and H2O are
A)hydrogen bonds.
B)dispersion forces.
C)covalent bonds.
D)dipolar forces.
A)hydrogen bonds.
B)dispersion forces.
C)covalent bonds.
D)dipolar forces.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
43
Polar substances tend to dissolve in non-polar solvents.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
44
Express the following concentration of solution in terms of molarity: 3.00 L of solution contains 1.75 mol of solute.
A)5.25 M
B)0.583 M
C)1.71 M
D)too little information to know
A)5.25 M
B)0.583 M
C)1.71 M
D)too little information to know

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following would be considered a strong electrolyte?
A)salt
B)sugar
C)acetic acid
D)all of the choices
A)salt
B)sugar
C)acetic acid
D)all of the choices

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
46
When sodium acetate dissolves in water,the following reaction occurs. 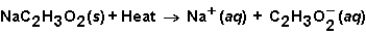 This solution could be used
This solution could be used
A)in a hot pack.
B)in a cold pack.
C)either a hot pack or cold pack.
D)neither a hot pack or cold pack.
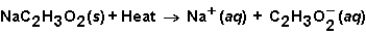 This solution could be used
This solution could be usedA)in a hot pack.
B)in a cold pack.
C)either a hot pack or cold pack.
D)neither a hot pack or cold pack.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
47
Drinking water can be purified by which of the following methods?
A)reverse osmosis
B)distillation
C)ion exchange
D)all of the choices
A)reverse osmosis
B)distillation
C)ion exchange
D)all of the choices

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
48
A solution is prepared at 75 °C by dissolving 50.0 g of A in 100 g of water.When this solution is cooled to 15 °C what happens based on the following solubility plot for A. 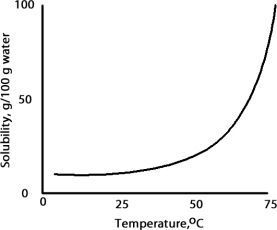
A)Solid A crystallizes and the solution is unsaturated.
B)Solid A dissolves and the solution is supersaturated.
C)Solid A crystallizes and the solution is saturated.
D)Solid A dissolves and the solution is unsaturated.

A)Solid A crystallizes and the solution is unsaturated.
B)Solid A dissolves and the solution is supersaturated.
C)Solid A crystallizes and the solution is saturated.
D)Solid A dissolves and the solution is unsaturated.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
49
You discover that your roommate has left a half-full bottle of cola on the kitchen counter without replacing the cap - AGAIN!.Not surprisingly,the cola is now flat (all carbonation is gone).Why did this happen?
A)The solubility of the carbon dioxide gas in the cola has decreased due to it being left at a lower pressure.
B)Warm bottles of cola will lose their carbonation faster than cold ones,even if the lid had been left on.
C)Oxygen in the atmosphere reacts with the cola,eliminating the carbonation.
D)The solubility of the carbon dioxide gas diffuses through the wall of the bottle.
A)The solubility of the carbon dioxide gas in the cola has decreased due to it being left at a lower pressure.
B)Warm bottles of cola will lose their carbonation faster than cold ones,even if the lid had been left on.
C)Oxygen in the atmosphere reacts with the cola,eliminating the carbonation.
D)The solubility of the carbon dioxide gas diffuses through the wall of the bottle.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following correctly arranges 1.00 M solutions of the strong electrolytes in order of increasing boiling point (lowest to highest)?
A)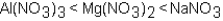
B)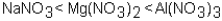
C)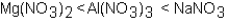
D)All have the same boiling point.
A)
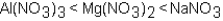
B)
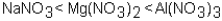
C)
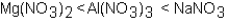
D)All have the same boiling point.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
51
A solution is prepared at 75 °C by dissolving 50.0 g of A in 100 g of water.Which of the following correctly classifies this solution based on the solubility chart for A given below? 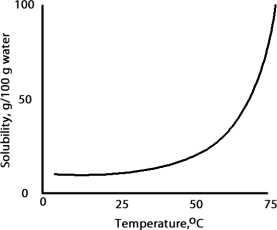
A)saturated
B)unsaturated
C)supersaturated
D)immiscible

A)saturated
B)unsaturated
C)supersaturated
D)immiscible

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
52
Citric acid,a natural food preservative,accounts for the tartness of citrus fruits.It is shown below.About 730 g of this material can be dissolved in water,making a liter of solution.However,only about 1.5% of it dissociates.As such,it would be considered a _____. 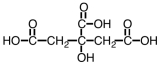
A)nonelectrolyte
B)strong electrolyte
C)pseudoelectrolyte
D)weak electrolyte

A)nonelectrolyte
B)strong electrolyte
C)pseudoelectrolyte
D)weak electrolyte

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
53
An ion in solution that is surrounded by water is a(n)____ ion.
A)hydrated
B)osmotic
C)saturated
D)colloid
A)hydrated
B)osmotic
C)saturated
D)colloid

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following is not considered a colligative property?
A)vapor pressure
B)boiling point
C)conductivity
D)freezing point
A)vapor pressure
B)boiling point
C)conductivity
D)freezing point

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
55
The cleaning action of soaps and detergents is attributable to
A)their ionic character.
B)the long hydrocarbon tail in their structure.
C)their ability to dissociate into ions.
D)all of the above.
A)their ionic character.
B)the long hydrocarbon tail in their structure.
C)their ability to dissociate into ions.
D)all of the above.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
56
When making some iced tea,you find that you can dissolve 100 grams of table sugar in a liter of tea at 20 oC.Based on this,what did you learn?
A)Based on the solubility,tea must be a nonpolar solvent.
B)The solubility of table sugar in tea at 20 oC is 100 g/L
C)Table sugar must be a strong electrolyte.
D)You can dissolved 100 grams of sugar in tea at 20 oC
A)Based on the solubility,tea must be a nonpolar solvent.
B)The solubility of table sugar in tea at 20 oC is 100 g/L
C)Table sugar must be a strong electrolyte.
D)You can dissolved 100 grams of sugar in tea at 20 oC

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
57
A colligative property
A)will only occur for aqueous solutions.
B)depends only on the number of species added to a solution.
C)results from increasing vapor pressure as a solute is added to a solvent.
D)All of the choices.
A)will only occur for aqueous solutions.
B)depends only on the number of species added to a solution.
C)results from increasing vapor pressure as a solute is added to a solvent.
D)All of the choices.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
58
During a surgical procedure,a wound was accidentally irrigated with deionized water,instead of normal saline.What would one expect to happen?
A)There would be a net movement of water into the exposed tissue.
B)Water would move out of the exposed tissue.
C)Sodium ions would migrate out of the exposed cells.
D)There would be no net effect since the solution was added externally.
A)There would be a net movement of water into the exposed tissue.
B)Water would move out of the exposed tissue.
C)Sodium ions would migrate out of the exposed cells.
D)There would be no net effect since the solution was added externally.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
59
When a patient's blood electrolyte levels are evaluated,sodium,chloride and bicarbonate ions are commonly measured and the difference in the total positive and negative charges calculated.This difference is called the
A)ionic ratio.
B)isoelectric point.
C)electromotive constant.
D)anion gap.
A)ionic ratio.
B)isoelectric point.
C)electromotive constant.
D)anion gap.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
60
One test to determine if a mixture is a true solution or a colloid is ____.
A)physical state of the mixture
B)boiling point elevation
C)light scattering
D)color
A)physical state of the mixture
B)boiling point elevation
C)light scattering
D)color

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
61
Weight-volume percentage solutions must be made in 100 mL increments.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
62
The more soluble a substance is,the faster it will dissolve.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
63
Solvents and hydrated ions can usually pass though dialyzing membranes

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
64
The solubility of a substance can be measured in grams substance dissolved per liter of water.This is the same as expressing solubility in moles per liter.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
65
All ionic compounds are soluble in water.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
66
Oil should be a good solvent for polar compounds.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
67
Emulsions are destroyed by adding salt.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
68
Light scattering is an effective way to distinguish between true solutions and colloidal dispersions.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
69
Dialysis and osmosis are used for the same purposes.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
70
Rock candy (large table sugar crystals)can be produced by allowing a hot,saturated solution of sugar in water to cool off.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
71
Ionic compounds are generally insoluble in non-polar solvents.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
72
Dialysis can be used to separate solutions from colloids.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
73
Attractive forces between solute and solvent molecules are an important factor in solution formation.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
74
The %(w/v)of a solution is defined as: 


Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
75
There is a 12 M aqueous HCl solution in the stock room,but a 6 M solution is required for an experiment.Doubling the volume of a 12 M sample with water will produce a 6 M solution.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
76
A mixture of sand and water would be an example of a colloidal suspension.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
77
Colloids can be stabilized by emulsifying agents.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
78
Colloids are considered to be homogenous mixtures.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
79
Putting a celery stick in distilled water results in the uptake of water by the celery.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
80
The solubility of gases in water increases with increasing temperature.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck


