Exam 7: Solutions and Colloids
Exam 1: Matter, measurements, and Calculations92 Questions
Exam 2: Atoms and Molecules89 Questions
Exam 3: Electronic Structure and the Periodic Law86 Questions
Exam 4: Forces Between Particles89 Questions
Exam 5: Chemical Reactions88 Questions
Exam 6: The States of Matter88 Questions
Exam 7: Solutions and Colloids87 Questions
Exam 8: Reaction Rates and Equilibrium86 Questions
Exam 9: Acids, bases, and Salts86 Questions
Exam 10: Radioactivity and Nuclear Processes86 Questions
Exam 11: Organic Compounds: Alkanes87 Questions
Exam 12: Unsaturated Hydrocarbons88 Questions
Exam 13: Alcohols, phenols, and Ethers86 Questions
Exam 14: Aldehydes and Ketones86 Questions
Exam 15: Carboxylic Acids and Esters86 Questions
Exam 16: Amines and Amides85 Questions
Exam 17: Carbohydrates88 Questions
Exam 18: Lipids91 Questions
Exam 19: Proteins89 Questions
Exam 20: Enzymes88 Questions
Exam 21: Nucleic Acids and Protein Synthesis90 Questions
Exam 22: Nutrition and Energy for Life89 Questions
Exam 23: Carbohydrate Metabolism90 Questions
Exam 24: Lipid and Amino Acid Metabolism94 Questions
Exam 25: Body Fluids86 Questions
Select questions type
A solution is prepared at 75 °C by dissolving 50.0 g of A in 100 g of water.When this solution is cooled to 15 °C what happens based on the following solubility plot for A. 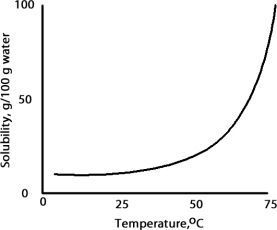
Free
(Multiple Choice)
4.9/5  (44)
(44)
Correct Answer:
C
Oil should be a good solvent for polar compounds.
Free
(True/False)
4.9/5  (41)
(41)
Correct Answer:
False
Which of the following correctly arranges 1.00 M solutions of the strong electrolytes in order of increasing boiling point (lowest to highest)?
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
B
When preparing an aqueous solution of a salt,one must always be able to observe some undissolved material to be sure that it is saturated.
(True/False)
4.9/5  (28)
(28)
A solution is made by dissolving 15.0 mL of oil in enough gasoline to give 50.0 mL of solution.What is the % (v/v)of oil in the solution?
(Multiple Choice)
4.9/5  (31)
(31)
The cleaning action of soaps and detergents is attributable to
(Multiple Choice)
4.7/5  (40)
(40)
A solution is prepared at 75 °C by dissolving 50.0 g of A in 100 g of water.Which of the following correctly classifies this solution based on the solubility chart for A given below? 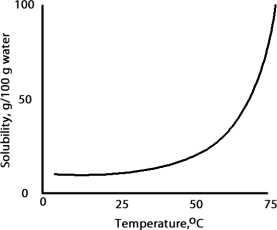
(Multiple Choice)
4.8/5  (32)
(32)
When solid NaOH is dissolved in water,the solution becomes hot.The solution process is
(Multiple Choice)
4.8/5  (32)
(32)
During a surgical procedure,a wound was accidentally irrigated with deionized water,instead of normal saline.What would one expect to happen?
(Multiple Choice)
4.9/5  (42)
(42)
What is the molarity of a solution containing 0.325 moles of solute in 250 mL of solution?
(Multiple Choice)
4.7/5  (36)
(36)
Drinking water can be purified by which of the following methods?
(Multiple Choice)
4.8/5  (32)
(32)
Two solutions with concentrations of 2% sugar and 4% sugar,respectively,are separated by a semipermeable membrane.During osmosis,there is a net flow of
(Multiple Choice)
4.7/5  (35)
(35)
An isotonic salt solution is 0.90% (w/w)NaCl in water.How many grams of NaCl are contained in 1.00 kg of such a solution?
(Multiple Choice)
4.9/5  (32)
(32)
A solution is made by dissolving a small amount of salt in a beaker of water.The water is referred to as the
(Multiple Choice)
4.9/5  (29)
(29)
A solution is prepared by adding 25.0 mL of 1.30 M AlCl3 solution to a flask,and then adding enough water to give a final volume of 200.0 mL.What is the molarity of the solution?
(Multiple Choice)
4.7/5  (32)
(32)
Showing 1 - 20 of 87
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)