Deck 9: Covalent Bonding: Orbitals
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/101
Play
Full screen (f)
Deck 9: Covalent Bonding: Orbitals
1
Consider the molecule and the following hybridization choices: 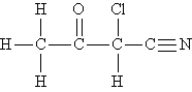
What is the hybridization of the carbon atom that is bonded to chlorine?
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3

What is the hybridization of the carbon atom that is bonded to chlorine?
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3
sp3
2
Consider the following Lewis structure: 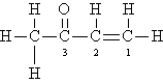 Which statement about the molecule is false?
Which statement about the molecule is false?
A)There are 10 sigma and 2 pi bonds.
B)C-2 is sp2 hybridized with bond angles of 120°.
C)Oxygen is sp3 hybridized.
D)This molecule contains 28 valence electrons.
E)There are some H-C-H bond angles of about 109° in the molecule.
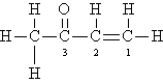 Which statement about the molecule is false?
Which statement about the molecule is false?A)There are 10 sigma and 2 pi bonds.
B)C-2 is sp2 hybridized with bond angles of 120°.
C)Oxygen is sp3 hybridized.
D)This molecule contains 28 valence electrons.
E)There are some H-C-H bond angles of about 109° in the molecule.
Oxygen is sp3 hybridized.
3
Consider the molecule and the following hybridization choices: 
What is the hybridization of the carbon atom that is double-bonded to oxygen?
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3

What is the hybridization of the carbon atom that is double-bonded to oxygen?
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3
sp2
4
The hybridization of the central atom in I3- is:
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
5
The hybridization of the central atom in XeF5+ is:
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
6
The hybridization of I in IF4- is
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
7
Consider the molecule and the following hybridization choices: 
What is the hybridization of the oxygen atom?
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3

What is the hybridization of the oxygen atom?
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
8
Consider the molecule and the following hybridization choices: 
What is the hybridization of the nitrogen atom?
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3

What is the hybridization of the nitrogen atom?
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
9
The hybridization of Cl in ClF2+ is
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
10
The hybridization of the carbon atom in the cation CH3+ is:
A)sp2
B)sp3
C)dsp
D)sp
E)none of these
A)sp2
B)sp3
C)dsp
D)sp
E)none of these

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
11
Atoms that are sp2 hybridized form ____ pi bond(s).
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
12
Consider the following Lewis structure: 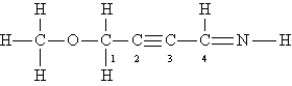 What is the hybridization of the atoms O,C-1,C-2,and C-4?
What is the hybridization of the atoms O,C-1,C-2,and C-4?
A)sp3 sp3 sp sp2
B)sp sp3 sp sp
C)sp sp2 sp sp2
D)sp2 sp3 sp2 sp3
E)sp3 sp sp sp2
O C-1 C-2 C-4
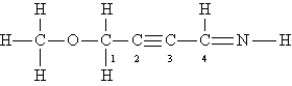 What is the hybridization of the atoms O,C-1,C-2,and C-4?
What is the hybridization of the atoms O,C-1,C-2,and C-4?A)sp3 sp3 sp sp2
B)sp sp3 sp sp
C)sp sp2 sp sp2
D)sp2 sp3 sp2 sp3
E)sp3 sp sp sp2
O C-1 C-2 C-4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following does not contain at least one pi bond?
A)H2CO
B)CO2
C)C2H4
D)C2H6
E)All of the above (A-D)contain at least one pi bond.
A)H2CO
B)CO2
C)C2H4
D)C2H6
E)All of the above (A-D)contain at least one pi bond.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
14
The hybridization of the central atom in O3 is:
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
15
Which statement about N2 is false?
A)It is a gas at room temperature.
B)The oxidation state is +3 on one N and -3 on the other.
C)It has one sigma and two pi bonds between the two atoms.
D)It can combine with H2 to form NH3.
E)It has two pairs of nonbonding electrons.
A)It is a gas at room temperature.
B)The oxidation state is +3 on one N and -3 on the other.
C)It has one sigma and two pi bonds between the two atoms.
D)It can combine with H2 to form NH3.
E)It has two pairs of nonbonding electrons.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following molecules contains a central atom with sp2 hybridization?
A)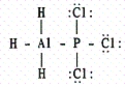
B)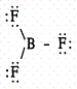
C)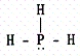
D)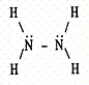
E)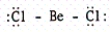
A)
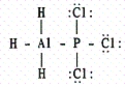
B)
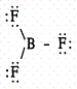
C)
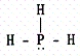
D)

E)

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
17
The hybridization of the central atom,Al,in AlBr3 is
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
18
The hybridization of the central atom in ClF2+ is:
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
19
What hybridization is predicted for the nitrogen atom in the NO3- ion?
A)sp2
B)sp3
C)dsp3
D)d2sp3
E)none of these
A)sp2
B)sp3
C)dsp3
D)d2sp3
E)none of these

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
20
In the molecule C2H4 the valence orbitals of the carbon atoms are assumed to be
A)not hybridized
B)sp hybridized
C)sp2 hybridized
D)sp3 hybridized
E)dsp hybridized
A)not hybridized
B)sp hybridized
C)sp2 hybridized
D)sp3 hybridized
E)dsp hybridized

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
21
The hybridization of Se in SeF6 is
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
22
Tetracyanoethylene has the skeleton shown below: 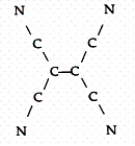 From its Lewis structure determine the following:
From its Lewis structure determine the following:
How many sigma and pi bonds are in the molecule?
A)4 sigma and 5 pi
B)6 sigma and 8 pi
C)9 sigma and 8 pi
D)9 sigma and 9 pi
E)5 sigma and 8 pi
 From its Lewis structure determine the following:
From its Lewis structure determine the following:How many sigma and pi bonds are in the molecule?
A)4 sigma and 5 pi
B)6 sigma and 8 pi
C)9 sigma and 8 pi
D)9 sigma and 9 pi
E)5 sigma and 8 pi

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
23
Consider the molecule C2H4.The hybridization of each C atom is
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following substances contains two pi bonds?
A)C2H4
B)C3H8
C)C2H2
D)C2H6
E)CH4
A)C2H4
B)C3H8
C)C2H2
D)C2H6
E)CH4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
25
Tetracyanoethylene has the skeleton shown below:  From its Lewis structure determine the following:
From its Lewis structure determine the following:
How many of the atoms are sp2 hybridized?
A)2
B)4
C)6
D)8
E)10
 From its Lewis structure determine the following:
From its Lewis structure determine the following:How many of the atoms are sp2 hybridized?
A)2
B)4
C)6
D)8
E)10

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
26
Consider the skeletal structure shown below:
N-C-C-N
Draw the Lewis structure and answer the following:
How many pi bonds does the molecule contain?
A)0
B)2
C)4
D)6
E)7
N-C-C-N
Draw the Lewis structure and answer the following:
How many pi bonds does the molecule contain?
A)0
B)2
C)4
D)6
E)7

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
27
Tetracyanoethylene has the skeleton shown below:  From its Lewis structure determine the following:
From its Lewis structure determine the following:
How many nonbonded electron pairs are in the molecule?
A)0
B)2
C)4
D)5
E)8
 From its Lewis structure determine the following:
From its Lewis structure determine the following:How many nonbonded electron pairs are in the molecule?
A)0
B)2
C)4
D)5
E)8

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
28
The hybridization of Br in BrF3 is
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
29
Complete the Lewis structure for the following molecule: 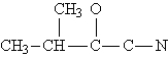 This molecule has __________ sigma and __________ pi bonds.
This molecule has __________ sigma and __________ pi bonds.
A)4,5
B)6,3
C)11,5
D)13,2
E)13,3
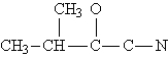 This molecule has __________ sigma and __________ pi bonds.
This molecule has __________ sigma and __________ pi bonds.A)4,5
B)6,3
C)11,5
D)13,2
E)13,3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
30
Tetracyanoethylene has the skeleton shown below:  From its Lewis structure determine the following:
From its Lewis structure determine the following:
How many of the atoms are sp hybridized?
A)2
B)4
C)6
D)8
E)10
 From its Lewis structure determine the following:
From its Lewis structure determine the following:How many of the atoms are sp hybridized?
A)2
B)4
C)6
D)8
E)10

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
31
The hybridization of the central atom in NO3- is
A)p3
B)sp2
C)sp3
D)sp
E)dsp2
A)p3
B)sp2
C)sp3
D)sp
E)dsp2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
32
Use the molecules below to answer the next three questions. 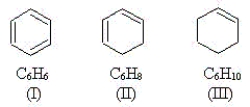
-Which molecule(s)have p orbitals that share an electron pair to create bonding?
A)I
B)II
C)III
D)all of the above
E)none of the above

-Which molecule(s)have p orbitals that share an electron pair to create bonding?
A)I
B)II
C)III
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
33
Whenever a set of equivalent tetrahedral atomic orbitals is required,an atom will adopt a set of sp3 orbitals.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
34
Use the molecules below to answer the next three questions. 
Which molecule(s)have equivalent C-C bonds throughout the molecule?
A)I
B)II
C)III
D)all of the above
E)none of the above

Which molecule(s)have equivalent C-C bonds throughout the molecule?
A)I
B)II
C)III
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
35
When a carbon atom has sp3 hybridization,it has
A)four bonds
B)three bonds and one bond
C)two bonds and two bonds
D)one bond and three bonds
E)four bonds
A)four bonds
B)three bonds and one bond
C)two bonds and two bonds
D)one bond and three bonds
E)four bonds

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
36
Consider the skeletal structure shown below:
N-C-C-N
Draw the Lewis structure and answer the following:
How many of the atoms are sp hybridized?
A)0
B)1
C)2
D)3
E)4
N-C-C-N
Draw the Lewis structure and answer the following:
How many of the atoms are sp hybridized?
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
37
Use the molecules below to answer the next three questions. 
Which molecule(s)have at least one carbon atom that is sp hybridized?
A)I
B)II
C)III
D)all of the above
E)none of the above

Which molecule(s)have at least one carbon atom that is sp hybridized?
A)I
B)II
C)III
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
38
The hybridization of the B in BH3 is sp3.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
39
The hybridization of the lead atom in PbCl4 is
A)dsp2
B)sp2
C)d2sp3
D)dsp3
E)none of these
A)dsp2
B)sp2
C)d2sp3
D)dsp3
E)none of these

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
40
A (pi)bond is the result of the
A)overlap of two s orbitals
B)overlap of an s orbital and a p orbital
C)overlap of two p orbitals along their axes
D)sidewise overlap of two parallel p orbitals
E)sidewise overlap of two s orbitals
A)overlap of two s orbitals
B)overlap of an s orbital and a p orbital
C)overlap of two p orbitals along their axes
D)sidewise overlap of two parallel p orbitals
E)sidewise overlap of two s orbitals

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
41
If four orbitals on one atom overlap four orbitals on a second atom,how many molecular orbitals will form?
A)1
B)4
C)8
D)16
E)none of these
A)1
B)4
C)8
D)16
E)none of these

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
42
If a molecule demonstrates paramagnetism,then :
I.The substance can have both paired and unpaired electrons.
II.The bond order is not a whole number.
III.It can be determined by drawing a Lewis structure.
IV.It must be an ion.
A)I,II
B)I,II,IV
C)II,III
D)I only
E)All of the above are correct.
I.The substance can have both paired and unpaired electrons.
II.The bond order is not a whole number.
III.It can be determined by drawing a Lewis structure.
IV.It must be an ion.
A)I,II
B)I,II,IV
C)II,III
D)I only
E)All of the above are correct.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following species is paramagnetic?
A)C2
B)O2
C)F2
D)Li2
E)none of these
A)C2
B)O2
C)F2
D)Li2
E)none of these

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following species has the largest dissociation energy?
A)O2
B)O2-
C)O22-
D)O2+
E)O22+
A)O2
B)O2-
C)O22-
D)O2+
E)O22+

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following diatomic molecules has a bond order of 2?
A)B2
B)C2
C)P2
D)F2
E)Li2
A)B2
B)C2
C)P2
D)F2
E)Li2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
46
For how many of the following does the bond order decrease if you add one electron to the neutral molecule? B2,C2,P2,F2
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
47
Order the following from shortest to longest bond: C2,B2,H2,N2
A)H2,N2,C2,B2
B)N2,C2,B2,H2
C)C2,N2,H2,B2
D)C2,B2,H2,N2
E)none of these
A)H2,N2,C2,B2
B)N2,C2,B2,H2
C)C2,N2,H2,B2
D)C2,B2,H2,N2
E)none of these

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following has the largest bond order?
A)N2
B)N2-
C)N22-
D)N2+
E)N22+
A)N2
B)N2-
C)N22-
D)N2+
E)N22+

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
49
As the bond order of a bond increases,the bond energy ______ and the bond length ______.
A)increases,increases
B)decreases,decreases
C)increases,decreases
D)decreases,increases
E)More information is needed to answer this question.
A)increases,increases
B)decreases,decreases
C)increases,decreases
D)decreases,increases
E)More information is needed to answer this question.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
50
Larger bond order means greater bond strength.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
51
When an electron pair is shared in the area centered on a line joining the atoms,a (sigma)bond is formed.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
52
The electron configuration of a particular diatomic species is ( 2s)2( *2s)2( 2p)2( 2p)4( *2p)2.What is the bond order for this species?
A)3.5
B)3
C)2.5
D)2
E)1.5
A)3.5
B)3
C)2.5
D)2
E)1.5

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
53
The hybridization of a molecule is measured to determine the shape of the molecule.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
54
The configuration ( 2s)2( 2s*)2( 2py)1( 2px)1 is the molecular orbital description for the ground state of
A)Li2+
B)Be2
C)B2
D)B22-
E)C2
A)Li2+
B)Be2
C)B2
D)B22-
E)C2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
55
Which charge(s)on an O2 ion would give a bond order of 2.5?
A)-2
B)-1
C)+1
D)two of these
E)none of these
A)-2
B)-1
C)+1
D)two of these
E)none of these

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
56
What is the bond order of He2+?
A)0
B)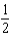
C)1
D)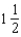
E)2
A)0
B)
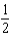
C)1
D)
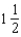
E)2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
57
The fact that O2 is paramagnetic can be explained by
A)the Lewis structure of O2
B)resonance
C)a violation of the octet rule
D)the molecular orbital diagram for O2
E)hybridization of atomic orbitals in O2
A)the Lewis structure of O2
B)resonance
C)a violation of the octet rule
D)the molecular orbital diagram for O2
E)hybridization of atomic orbitals in O2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
58
For which of the following diatomic molecules would the bond order become greater if an electron is removed (i.e. ,if the molecule is converted to the positive ion in its ground state)?
A)B2
B)C2
C)P2
D)F2
E)Na2
A)B2
B)C2
C)P2
D)F2
E)Na2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
59
When comparing Be2 and H2:
I.Be2 is more stable because it contains both bonding and antibonding valence electrons.
II.H2 has a higher bond order than Be2.
III.H2 is more stable because it only contains 1s electrons.
IV.H2 is more stable because it is diamagnetic,whereas Be2 is paramagnetic.
A)I,II
B)III only
C)II,III
D)II,III,IV
E)III,IV
I.Be2 is more stable because it contains both bonding and antibonding valence electrons.
II.H2 has a higher bond order than Be2.
III.H2 is more stable because it only contains 1s electrons.
IV.H2 is more stable because it is diamagnetic,whereas Be2 is paramagnetic.
A)I,II
B)III only
C)II,III
D)II,III,IV
E)III,IV

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
60
How many of the following: F2,B2,O2,N2 ,are paramagnetic?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
61
According to MO theory,F2 should be diamagnetic.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
62
The H2- ion is more stable than H2 since it has an additional electron to produce a net lowering of energy.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following has the greatest bond strength?
A)B2
B)O2-
C)CN-
D)O2+
E)NO-
A)B2
B)O2-
C)CN-
D)O2+
E)NO-

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following statements is false?
A)C2 is paramagnetic.
B)C2 is diamagnetic.
C)The carbon-carbon bond in C22- is stronger than the one in CH3CH3.
D)The carbon-carbon bond in C22- is shorter than the one in CH3CH3.
E)Two of the above.
A)C2 is paramagnetic.
B)C2 is diamagnetic.
C)The carbon-carbon bond in C22- is stronger than the one in CH3CH3.
D)The carbon-carbon bond in C22- is shorter than the one in CH3CH3.
E)Two of the above.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following statements about the species CN- is false?
A)It is paramagnetic.
B)The total number of electrons is 14.
C)Its bond order is 3.
D)It has two pi bonds.
E)All of these are true.
A)It is paramagnetic.
B)The total number of electrons is 14.
C)Its bond order is 3.
D)It has two pi bonds.
E)All of these are true.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
66
In the molecular orbital description of CO:
A)The highest energy electrons occupy antibonding orbitals.
B)Six molecular orbitals contain electrons.
C)There are two unpaired electrons.
D)The bond order is 3.
E)All of the above are false.
A)The highest energy electrons occupy antibonding orbitals.
B)Six molecular orbitals contain electrons.
C)There are two unpaired electrons.
D)The bond order is 3.
E)All of the above are false.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
67
A species has the following MO configuration: ( 1s)2( 1s*)2( 2s)2( 2s*)2( 2p)2( 2p)2
This substance is
A)paramagnetic with one unpaired electron
B)paramagnetic with two unpaired electrons
C)paramagnetic with three unpaired electrons
D)paramagnetic with four unpaired electrons
E)diamagnetic
This substance is
A)paramagnetic with one unpaired electron
B)paramagnetic with two unpaired electrons
C)paramagnetic with three unpaired electrons
D)paramagnetic with four unpaired electrons
E)diamagnetic

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following statements about the molecule BN is false?
A)It is paramagnetic.
B)Its bond order is 2.
C)The total number of electrons is 12.
D)It has two pi bonds.
E)All of these are true.
A)It is paramagnetic.
B)Its bond order is 2.
C)The total number of electrons is 12.
D)It has two pi bonds.
E)All of these are true.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following electron distributions among the molecular orbitals best describes the NO molecule?
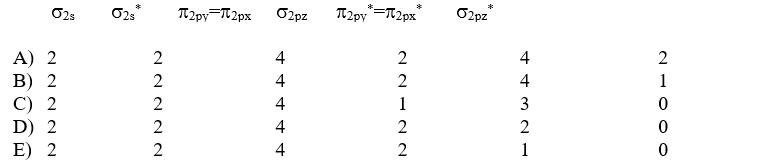


Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
70
How many unpaired electrons in the F22+ ion are based on molecular orbital theory? The order of the molecular orbitals are ( 2s)( *2s)( 2p)( 2p)( *2p)( *2p).
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
71
Consider the molecular orbital description of the NO- anion.Which of the following statements is false?
A)NO- is paramagnetic.
B)NO- is isoelectronic with CO.
C)The bond energy in NO+ is greater than the bond energy in NO-.
D)The bond order in NO- is 2.
E)Statements A through D are false.
A)NO- is paramagnetic.
B)NO- is isoelectronic with CO.
C)The bond energy in NO+ is greater than the bond energy in NO-.
D)The bond order in NO- is 2.
E)Statements A through D are false.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
72
What is the bond order of Ne2?
A)0
B)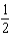
C)1
D)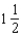
E)2
A)0
B)
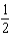
C)1
D)
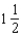
E)2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the nitrogen-containing molecules below is paramagnetic in its lowest energy state?
A)N2
B)NO
C)NH3
D)N2H4
E)none of these
A)N2
B)NO
C)NH3
D)N2H4
E)none of these

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
74
For how many of the following does bond order decrease if you take away one electron from the neutral molecule? B2,C2,P2,F2
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following statements is incorrect?
A)For the molecule NO,the molecular orbital model is preferred over the localized electron model because NO contains an unpaired electron.
B)Electrons in antibonding orbitals will cause a molecule to be paramagnetic.
C)According to the molecular orbital model,when bonding occurs between hydrogen and bromine to make HBr,the 1s orbital of the hydrogen atom no longer exists.
D)Antibonding electrons are higher in energy than the atomic orbitals from which they came.
E)At least two of the above are incorrect.
A)For the molecule NO,the molecular orbital model is preferred over the localized electron model because NO contains an unpaired electron.
B)Electrons in antibonding orbitals will cause a molecule to be paramagnetic.
C)According to the molecular orbital model,when bonding occurs between hydrogen and bromine to make HBr,the 1s orbital of the hydrogen atom no longer exists.
D)Antibonding electrons are higher in energy than the atomic orbitals from which they came.
E)At least two of the above are incorrect.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following molecules or ions is not paramagnetic in its ground state?
A)O2
B)O2+
C)B2
D)NO
E)F2
A)O2
B)O2+
C)B2
D)NO
E)F2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following has a bond order of 1.5?
A)O2+
B)N2
C)O2-
D)C2
E)none of these
A)O2+
B)N2
C)O2-
D)C2
E)none of these

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
78
Paramagnetism is associated with paired electrons.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
79
What is the bond order of C2+?
A)0
B)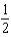
C)1
D)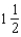
E)2
A)0
B)
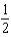
C)1
D)
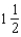
E)2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following has the shortest bond length?
A)O22-
B)O2
C)O2-
D)O2+
E)Two of these have the shortest bond length.
A)O22-
B)O2
C)O2-
D)O2+
E)Two of these have the shortest bond length.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck


